> ENC Master > Climate Encyclopaedia > Clouds & Particles > basics > 1. Clouds > - Water in the atmosphere
> ENC Master > Climate Encyclopaedia > Clouds & Particles > basics > 1. Clouds > - Water in the atmosphere
 |
|
|
|
Clouds & ParticlesBasics |
Water in the atmosphereWater is the only substance that can exist naturally under 3 forms: liquid (oceans, rivers, lakes...), solid (ice, snow...) and gaseous (water vapour). In the atmosphere, there is not only air, but also water vapor, that is invisible and odourless. In the atmosphere, water account for less than 0.001% of the Earth's water, but is an important player on climate. Let's see the role of water in the atmosphere and what are clouds made of. |
|
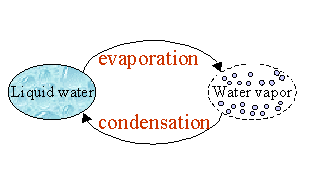
When you drink water, it is in a liquid form, called liquid state; when you crunch an ice cube, your teeth know that water is solid. But water can also be in a gaseous state, i.e. water is under the form of free molecules, that are collectively known as water vapour or moisture. When the water in the liquid state change to vapour state, that is called evaporation. That is what happens when your linen dries. Or when you use an hair-drier : your hair was wet and it is now dry. Water has disappeared?! In fact, it is still in your bathroom, but in the air. Due to the high temperature of the hair-dryer, liquid water has changed to vapour, it has evaporated! |
|
Condensation is the "contrary": it is the process of turning vapor into liquid water. After having a bath, the bathroom is filled with steam, or water vapour. The warm steam condenses on the cold bathroom mirror, returning to it's liquid state: you can see droplets on the mirror. |
 |
|
1. Condensation and evaporation. Author: J. Gourdeau |
You could never walk on a cloud as it is only water in the air: clouds form as a result of water vapour turning into droplets or ice crystals light enough to float in the air. When air containing water vapour is cooled to its saturation point, then the water vapour condenses into visible water droplets, called clouds. In other words, when the temperature is lowered, the saturation point corresponds to the moment at which condensation occurs or dew forms. We'll see later the clouds formation processes. In order to form clouds, water vapour also needs tiny particles in the atmosphere to condense on, called Cloud Condensation Nuclei. We will discuss how these particles help cloud formation in Unit 2. |
|
|
Precipitation is the name of water that falls out of clouds: it can be rain, or snow, or hail... In some clouds the tiny water droplets come together by collision to make bigger drops. As the drops become bigger and bigger (volume increases about a million times) they become too heavy for the air to support and they fall as rain. |
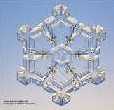
Clouds with surrounding air temperature below 0°C are made of ice crystals. These ice crystals that are formed near droplets of super-cooled water (water that remains as liquid even below 0°C), increase in size when water vapour from cloud droplets is deposited on the ice crystals. As the ice crystals fall, they may collide and this makes the ice crystals heavier. When the ice crystals are too heavy to float in the air, it will fall to the ground. The crystals become snow, or raindrops if they fall through air that is hotter than 0°C. |
|
3. Ice crystals (© Rasmussen and Libbrecht , Y. Furukawa, www.snowcrystals.com) |
About this page... |