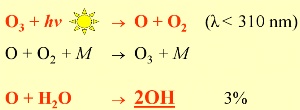> English > Climate Encyclopaedia > Lower Atmosphere > more > 1. Oxidants & Observation > - oxidation & OH radicals
> English > Climate Encyclopaedia > Lower Atmosphere > more > 1. Oxidants & Observation > - oxidation & OH radicals
|
Lower AtmosphereRead more |
Oxidation in the AtmosphereMany chemical compounds are emitted into the atmosphere but removal processes prevent them accumulating in the air. Species are removed by dry deposition of gases or particles or can be incorporated into rain and removed by wet deposition. For gas phase organic chemicals, removal is easiest if they are first oxidised to a less volatile, water soluble form.
|
|
Oxidation in a chemical sense does not necessarily mean a reaction with oxygen containing compounds, it is rather the loss of electrons. However, in the air, oxidation does generally involve the reaction of a chemical species with an oxygen containing compound. The three most important oxidising species in the air are: the hydroxyl radical OH Hydroperoxy radicals (HO2) are also important and the sum of HO2 and OH is sometimes referred to as HOx. The most important oxidising species is the hydroxyl radical (OH). It is extremely reactive and able to oxidise most of the chemicals found in the troposphere. The hydroxyl radical is therefore known as the 'detergent of the atmosphere'.
|
|
How is OH formed?OH governs atmospheric chemistry during the day since its formation depends on radiation from the Sun. The initial reaction (shown above) involves the breakdown (photolysis) of ozone by solar radiation with wavelengths less than 310 nm. The oxygen atom (O) formed then reacts with water to form OH. This reaction mechanism is why a small amount of ozone is essential in the troposphere. Other sources of OH are:
|
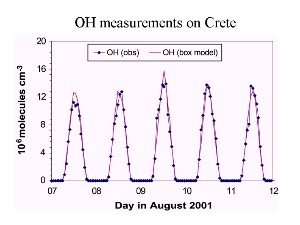
How much OH is formed?Since OH is an extremely reactive radical it reacts as soon as it is formed. It's lifetime is less than a second. This means the concentration is extremely low, in the range of 1x105 to 2x107 molecules cm-3. At sea level pressure this is equivalent to a mixing ratio of 0.01 - 1 ppt (pmol/mol). Only one of 1000 billion to 100,000 billion molecules in the air is OH. Since it's formation depends on water vapour, the concentration of OH tends to decrease with altitude as the air becomes cooler and drier. |
|
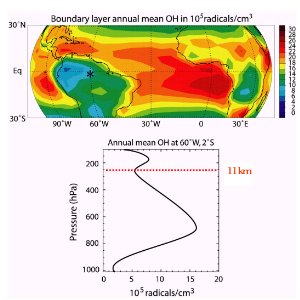
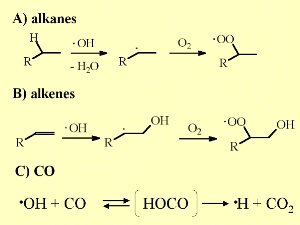
Hydroxyl radical concentrations not only decrease with altitude but also decrease with latitude since both the water vapour concentrations and sunlight intensity decrease as you move towards the poles. How does OH react?The figure on the right shows low levels of OH near the ground over the tropical rainforest. Why is this the case? Plants emit organic gases with isoprene being the most abundant. This isoprene reacts with OH, removing it from the air, and forming water and a reactive organic radical (R). OH has a strong tendancy to remove (abstract) a hydrogen atom from organic species (RH) whenever possible. The organic radical (R) then reacts with oxygen (O2) to form organic peroxides (RO2). These compounds are an essential part of the ozone formation cycle.
|
|
|
On a global scale, OH reacts primarily with carbon monoxide (40%) to form carbon dioxide. Around 30% of the OH produced is removed from the atmosphere in reactions with organic compounds and 15% reacts with methane (CH4). The remaining 15% reacts with ozone (O3), hydroperoxy radicals (HO2) and hydrogen gas (H2).
|
|
The oxidation of carbon monoxide and methane by OH are very important as they are the major ways by which OH is removed from the atmosphere. Reaction of OH with alkenes, a special class of organic compounds, is also very important as this reaction results in the formation of peroxides. |
OH is the most important oxidant in the atmosphere. However OH concentrations are close to zero during the night since sunlight is needed for its formation. So during dark periods and during night-time, ozone and nitrate radical (NO3) chemistry become important.
|
|
* The mixing ratio ppb (1 molecule in 1 billion molecules of air) or ppm (1 molecule in 1 million molecules of air) is often used in scientific publications as well as in other literature on the atmosphere and climate. Because of this we use it here in the Climate Encyclopaedia. However, the correct unit is 1 nmol mol-1 (equivalent to 1 ppb) or 1 Ámol m-1 (for 1 ppm) since mole is the standard unit of concentration.About this page:author: Dr. Elmar Uherek - MPI for Chemistry, Mainz
|