> English > Climate Encyclopaedia > Clouds and Particles > more > 1. What happens in clouds? > - Cloud chemistry
> English > Climate Encyclopaedia > Clouds and Particles > more > 1. What happens in clouds? > - Cloud chemistry
|
Clouds & ParticlesMore |
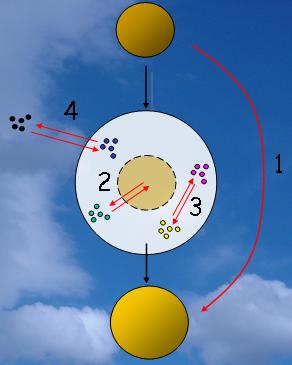
Cloud chemistryClouds are not an inert mixture of water droplets (or ice crystals) and particles. Particles that allow clouds to form are called cloud condensation nuclei (CCN). These particles have different chemical compositions depending on their origin. They can have natural sources and originate from the deserts, from the oceans, from volcanoes or from living organisms or they can come from human processes. The cloud also contains gases which can change the chemical composition of the droplets. So a cloud is far from inert!
|
The particle inside the droplet
The water soluble fraction of an aerosol particle governs whether it can take up water and grow into a droplet. The chemical composition of CCN particles controls the initial chemical composition of a cloud droplet as its soluble content dissolves in the condensed water. The less water soluble particles, for example soil dust, pollen and particles from biomass burning, remain in the surrounding air.
Most clouds don't lead to rain and simply evaporate. As a result of in-cloud chemical reactions, the particles which remain after the water evaporates have a different chemical composition to those which entered the cloud in the first place.
|
 |
|
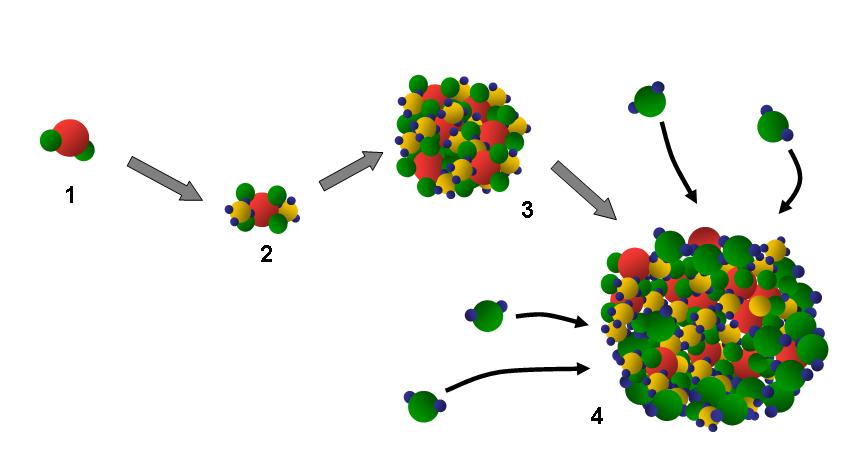
2. A sulphur dioxide molecule (1) reacts with ammonia in the air to form to ammonium sulphate (2). This then grows to form an ammonium sulphate particle (3). These particles are hygroscopic, meaning they rapidly grow in the presence of water (4). Author: J. Gourdeau.
|
The atmospheric gases around the dropletWhether a chemical species stays in the gas phase or is absorbed by the water droplet is determined by the Henry’s law equilibrium: A(aq) = HA PA where: Some species go back to the gas phase and move away from the drop. Others, once captured, remain associated with the aqueous phase unless total evaporation occurs.
|
Reactions inside the dropletAt least a hundred chemical reactions take place in a droplet. These reactions can change the acidity of the rainfall resulting in acid rains which are hazardous to plants and animals living in lakes and streams and can contribute to the deterioration of buildings. The main chemical species involved in acid rain are sulphuric (H2SO4) and nitric acids (HNO3).
|
|
|
All this complex chemistry modifies not only the cloud itself but also the atmosphere around the cloud. Only about one cloud in every seven results in rain and, as a result, a single particle acting as a CCN undergoes between 10 and 25 evaporation-condensation cycles before it reaches the ground.
|
About this pageauthor: Dr. Justine Gourdeau - LaMP, Clermont-Ferrand, France
|