
> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - CFC & HCFC
|
|
 |
Upper Atmosphere
Read more |
Chlorofluorocarbon's and HCFC's
The investigation of the ozone hole and the measures to ban chlorofluorocarbons (CFC's) world-wide is probably the most important example of a successful environmental protection policy. In this unit we look at the role played by CFC's, what role they are still playing and how they are being replaced.
|
 |
 |
|
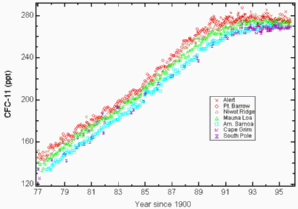
1. The long term trend of CFC-11 since 1977: Mixing ratios in ppt at different measurement stations. Source: NOAA/CMDL. Please click to enlarge! (15 K)
|
|
 |
Trends in CFC concentrations
Since CFC's are non-toxic and chemically inert gases in the troposphere they were used extensively in the past. However, it was found subsequently that CFC's are greenhouse gases with very high global warming potentials and they have a very long atmospheric lifetime. Their long lifetime means that they can be transported slowly to the stratosphere. Although CFC's were banned within a few years of the Montreal Protocol in 1987, their long lifetime means that it will take many more years before their atmospheric concentrations level out and then decrease.
|
 |
 |
|
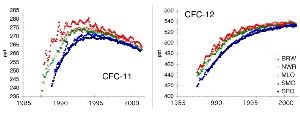
2. The recent trends for CFC-11 and CFC-12. Units are mixing ratios in ppt. Source: NOAA/CMDL. Please click to enlarge! (50 K)
|
|
 |
The maximum concentration at the ground for CFC-11 (which has a lifetime of 45 years) was reached in 1994, the maximum for CFC-12 (lifetime 100 years) may be reached now. Concentrations in the Northern Hemisphere are slightly higher than those in the Southern Hemisphere, because mixing within a hemisphere is always faster than transport across the equator.
|
Comparison of CFC's and HCFC's
Global warming potentials (GWP's) are a measure of the relative radiative effect of a given substance compared to CO2, integrated over a chosen time horizon. More simply, the GWP tells us whether a compound is a stronger or weaker greenhouse gas than CO2 and by how much.
Ozone depletion potentials (ODP's) are relative numbers which compares how dangerous different compounds are for the ozone layer.
CFC's have a twofold negative impact on our climate - they destroy ozone in the stratosphere and act as greenhouse gases in the troposphere. The following table compares CFC's with the hydrofluorocarbons (HCFC's) which have replaced them.
|
|
|
Direct Global Warming Potentials (mass basis) relative to carbon dioxide (for gases for which the lifetimes have been adequately characterised). Data from IPCC TAR 2001 |
|

|
|
Gas |
Lifetime (years) |
Ozone
depletion
potential
(ODP) |
Global Warming Potential
Time horizon |
|
|
|
|
|
|
|
|
|
20 years |
100 years |
|

|
|
Carbon dioxide |
CO2 |
|
|
1 |
1 |
|
Methane |
CH4 |
12.0* |
|
62 |
23 |
|
Nitrous oxide |
N2O |
114* |
|
275 |
296 |
|
Chlorofluorocarbons |
|
CFC-11 |
CCl3F |
45 |
1.0 |
6300 |
4600 |
|
CFC-12 |
CCl2F2 |
100 |
0.82 |
10200 |
10600 |
|
CFC-13 |
CClF3 |
640 |
|
10000 |
14000 |
|
Hydrochlorofluorocarbons |
|
HCFC-21 |
CHCl2F |
2.0 |
|
700 |
210 |
|
HCFC-22 |
CHClF2 |
11.9 |
0.04 |
4800 |
1700 |
|
HCFC-123 |
CF3CHCl2 |
1.4 |
0.014 |
390 |
120 |
|
Hydrofluorocarbons |
|
HFC-23 |
CHF3 |
260 |
<0.0004 |
9400 |
12000 |
|
HFC-32 |
CH2F2 |
5.0 |
|
1800 |
550 |
|
HFC-41 |
CH3F |
2.6 |
|
330 |
97 |
|

|
|
* The values for CH4 and N2O are adjustment times including emission feedbacks on lifetimes.
Ozone depletion potentials are normalized model results from the WMO Scientific Assessment of ozone depletion in 1994.
| | |
|
It is obvious that the HCFC's have much shorter atmospheric lifetimes than the CFC's. This means that they are mainly broken down in the troposphere and the probability that they will reach the stratosphere and participate in ozone destruction is much lower. However, they still act as strong greenhouse gases in the troposphere and so can be regarded as a compromise but not the best solution to the problem.
|
About this page:
author: Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
scientific reviewer: Dr. Christoph Bruhl - Max Planck Institute for Chemistry, Mainz, Germany
educational proofreading: Michael Seesing - Uni Duisburg - 2003-08-07
last published: 2004-05-11
|
 > English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - CFC & HCFC
> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - CFC & HCFC

