> ENC Master > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Chlorine chemistry
> ENC Master > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Chlorine chemistry
 |
|
|
|
Higher AtmosphereRead more |
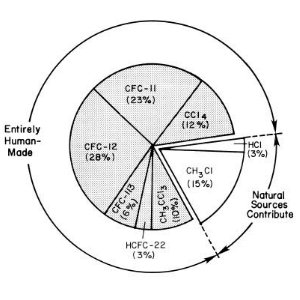
Chlorine chemistryPrimarily chlorine chemistry is driving the destruction of the ozone layer. With the industrial production of chlorofluorocarbons (CFCs) man brought a new chlorine source into the atmosphere. Now chlorine has six times the level it had from natural sources with fatal consequences for the ozone layer. However, conditions for the formation of the ozone hole are very special. Therefore a such drastic development has not been predicted.
|
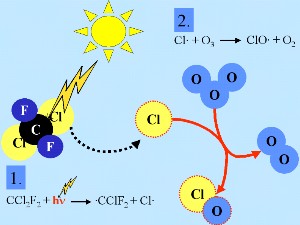
Stratospheric chlorine chemistry
|
|
But the initiating radical X |
|
ClO HCl and ClONO2 are so called 'reservoir species', because here chlorine is not active. They do not react with ozone. Normally they stay in the gas phase and can be slowly removed again from the stratosphere. Therefore in normal stratospheric gas phase chemistry, only slight ozone depletion is expected. But these species are transported with the mean circulation to the lower stratosphere in the polar winter area ... |
|
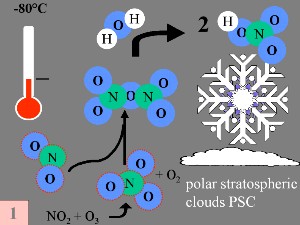
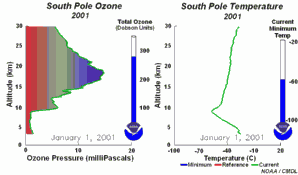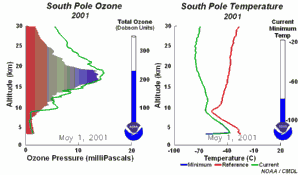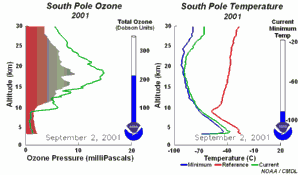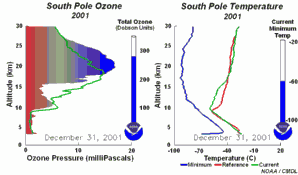
The special conditions of the Antarctic ozone holeDuring the polar night with temperatures of about -80°C, the very small amount of water available in the stratosphere is able to freeze and to form polar stratospheric ice clouds together with nitric acid (so called nitric acid trihydrate NAT). Now five key conditions can come together:
|
|
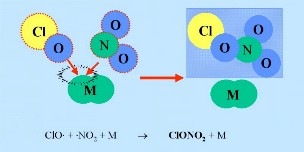
First: The nitrogen oxide catalysts (nitrogen oxide NO and nitrogen dioxide NO2), which help to convert ClO
thereby producing nitric acid HNO3 which is incorporated in the particles of the polar stratospheric clouds (PSC). |
|
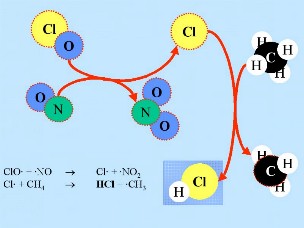
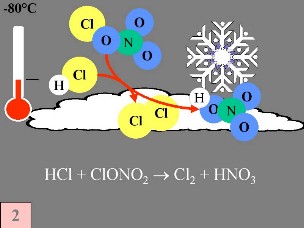
Second: On the surface of the PSC ice particles the 'reservoir species' for unreactive chlorine, HCl and ClONO2, react with each other to produce Cl2 and HNO3; the latter is immediately incorporated in the particles. Third: After the return of daylight at the end of the polar night, Cl2 is photolysed to produce 2 Cl
|
|
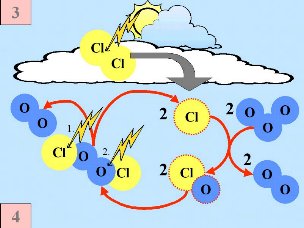
Fourth: The chlorine atoms start a catalytic chain of reactions, leading to the destruction of ozone as long as no nitrogen oxides are available to remove them. Note, that the speed of ozone destruction is quadratic in the chlorine (or ClO) concentration. Cl |
|
Fifth: Normally chlorine species as Cl
|
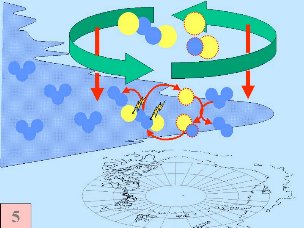
All five conditions have to come together, to form the ozone hole. This is why the major ozone depletion occurs over Antarctica and only in the Antarctic spring months September / October as soon as the sun rises after the polar night. In some years, we have comparable conditions over the Arctic in March and a little ozone hole forms over Northern Europe as well. Later in the year, the polar clouds dissolve, nitrogen oxides become available again, the vortex breaks together, chlorine radicals are removed and the ozone layer recovers.
|
|
M*: In any sort of reaction A + B -> C a third partner is needed, which takes away excess energy. Otherwise the product C would have the same energy than the sum of the educts A + B and directly react back. In most cases M is the nitrogen from the air N2.
|
About this page:author: Dr. Elmar Uherek - Max Panck Institute for Chemistry, Mainz
|