> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Chlorine chemistry
> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Chlorine chemistry
|
Upper AtmosphereRead more |
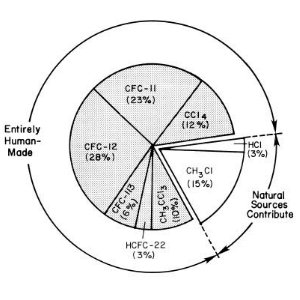
Chlorine chemistryIndustrial production and use of chlorofluorocarbons (CFC's) has introduced a new chlorine source into the atmosphere. Since chlorine chemistry is primarily responsible for ozone destruction, these CFC's have had fatal consequences for the ozone layer. However, because conditions required for the formation of the ozone hole are so very unusual, nobody predicted just how dangerous these compounds would be.
|
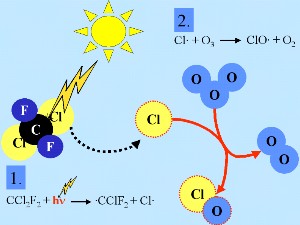
Stratospheric chlorine chemistry - the basicsGenerally, as for many other radicals (denoted X in the example below), chlorine radicals (Cl) are oxidised by ozone in the stratosphere to form XO (ClO) X + O3 -> XO + O2 This chain reaction drives ozone depletion. |
|
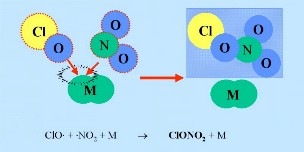
The initiating radical X (here Cl) is not necessarily recycled and Cl or ClO can also be removed in other reactions. Nitrogen oxides can react with ClO radicals to form so called "reservoir species" HCl and ClONO2 (shown in the dark blue box in the figure). An inert compound (M) is needed as part of the reaction to take away excess energy.
|
|
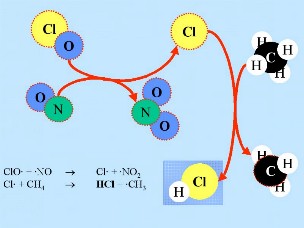
ClO + NO2 + M -> ClONO2 + M ClO + NO -> Cl + NO2 HCl and ClONO2 are known as resevoir species because the chlorine they contain is not active and the species don't react with ozone. They normally exist in the gas phase and are slowly removed from the stratosphere. In normal stratospheric gas phase chemistry, only slight ozone depletion is, therefore, expected. However, the resevoir species are transported down into the lower stratosphere in the winter as a result of the atmospheric circulation pattern.
|
|
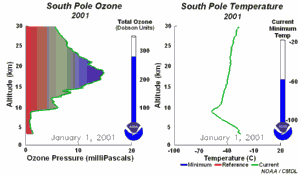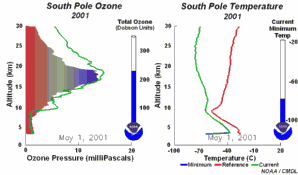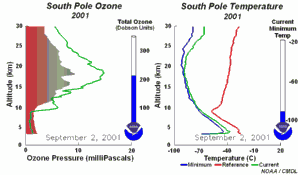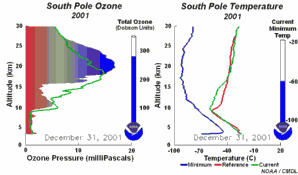
The special conditions of the Antarctic ozone holeDuring the polar night, air temperatures fall as low as -80 oC. Under these conditions, the small amount of water and nitric acid present in the stratosphere freeze (making nitric acid trihydrate) and form polar stratospheric ice clouds. Five key conditions can now come together:
|
|
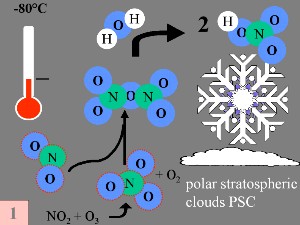
Firstly: Nitrogen oxide NO and nitrogen dioxide NO2, (which help to convert ClO into HCl, as shown above), are removed from the stratospheric gas phase through the reactions: NO + O3 -> NO2 + O2 The end product is nitric acid (HNO3), which is incorporated into the polar stratospheric clouds (PSC). |
|
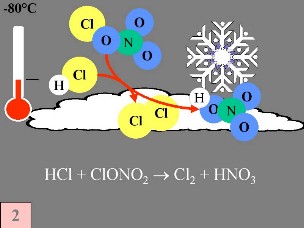
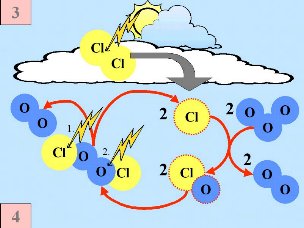
Secondly: HCl and ClONO2 react with each other on the surface of the polar stratospheric clouds to produce Cl2 and HNO3. This nitric acid is immediately incorporated into the ice particles of the cloud. Thirdly: When the sun rises at the end of the polar winter, Cl2 is broken down by solar radiation to produce two chlorine (Cl) radicals.
|
|
Fourthly: If there are no nitrogen oxides present, the chlorine radicals start a catalytic chain of reactions, leading to ozone destruction. Cl + O3 -> ClO + O2 |
|
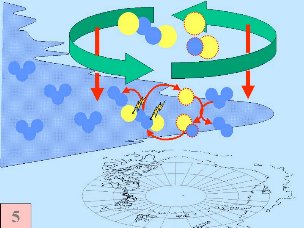
Fifthly: Normally chlorine species, such as Cl, ClO, and Cl2O2, are formed and concentrated in the upper stratosphere whereas ozone is found more in the lower stratosphere. Experts thought that ozone and ozone destroying chemicals would, therefore, only come together in border zones. As a result, ozone levels were not expected to decrease significantly although concentrations of chlorine containing compounds were rising in the stratosphere. However, nobody considered the polar vortex. This meteorologically stable vortex (circumpolar wind) with the pole more or less at its centre, transports chlorine rich air from the upper stratosphere to the ozone rich lower stratosphere allowing significant ozone destruction to occur.
|
All five conditions have to come together, to form the ozone hole. This is why the major ozone depletion occurs only over the poles (mainly Antarctica) and only in the spring as soon as the Sun rises after the polar winter. Later in the year as air temperatures increase, the polar clouds melt, nitrogen oxides become available again, the vortex breaks down preventing transport of reactive chlorine species to the lower stratosphere and the ozone layer recovers.
|
|
M: In any sort of reaction A + B -> C a third partner is needed, which takes away excess energy. Otherwise the product C would have the same energy than the sum of the reactants A + B and directly react back. In most cases M is nitrogen gas (N2) from the air.
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
|