> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Stratospheric ozone
> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - Stratospheric ozone
|
Upper AtmosphereRead more |
Stratospheric Ozone ChemistryDiscovery of the stratospheric ozone hole in 1985, led to a dramatic increase in ozone chemistry research. The following two chapters give an overview of the chemistry of the stratosphere in its historical frame.
|
Discovery of ozone and first measurementsOzone research is a rather old branch of atmospheric science. In 1840, the gas was baptised 'ozone' (the smelling) by the chemist Christian Friedrich Schönbein, who discovered that this substance was formed during electrical discharges. Very soon after, it was discovered that ozone is a natural component of air. The first method for measuring the gas was developed by Schönbein himself, and the method was subsequently improved at the Mount Souris Observatory in Paris. From this observatory we have the first time series of ozone data (1876 - 1910) and this data gives us the best idea of what atmospheric concentrations were like before the industrial revolution.
|
|
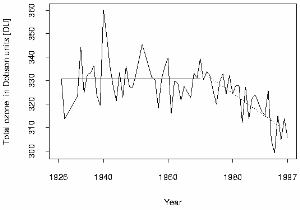
One of the first six Dobson spectrometers was used at Arosa in Switzerland by Paul Götz and from there we have the longest time series of total ozone column measurements in the world. The trend shows that the ozone layer has become thinner over time. Values below 300 DU have been measured recently at Hohenpeissenberg in Germany, a critical limit which makes better Sun protection necessary. In the 1930's, Götz showed that the ozone concentration maximum was likely to be below an altitude of 25 km and, as a result of his work, the ozone layer was located and its thickness measured.
|
|
|
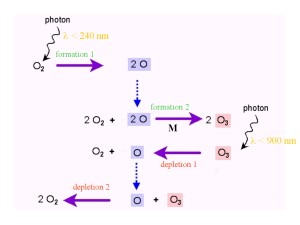
The Chapman ReactionsSo how is ozone produced and removed in the atmosphere? In 1929 and 1930, S. Chapman published the theory of ozone formation and destruction. The reactions proposed have since been confirmed as correct and are called the 'Chapman Cycle' or the 'Chapman Reactions'. These reactions show how oxygen and ozone are transformed into each other. Bonds between oxygen atoms are broken down by radiation from the Sun. More energy is required to break the O-O bond in O2 (wavelengths shorter than 240 nm) compared to the energy needed to break down O3 (wavelengths shorter than 900 nm). The formation and destruction reactions of ozone are in equilibrium - rates of formation and destruction are balanced - and the net result is a 'zero' reaction: 3 O2 -> 2 O3 and 2 O3 -> 3 O2
|
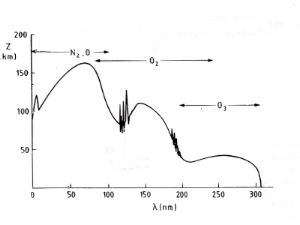
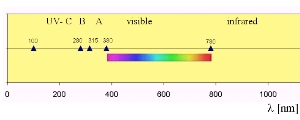
Absorption of ultra-violet radiationBecause bonds between atoms in molecules have varying strengths, it takes different amounts of energy to break them down. The bond between the two oxygen atoms in an oxygen molecule (O2) is really strong and can only be broken down if the molecule absorbs very high energy UV-C radiation. The bonds between the oxygen atoms in ozone are a bit weaker and can be broken down by absorption of slightly less energetic UV-B radiation. Absorption of this highly energetic, short wavelength, radiation by O2 and O3 in the upper atmosphere prevents it reaching the troposphere and destroying O2 and O3 there. Less energetic radiation (with longer wavelengths) reaches the surface of the Earth. |
|
|
A small fraction of UV-B radiation does reach the Earth's surface. The presence of this high energy radiation enables the hydroxy (OH) radical to form and this cleans the atmosphere of harmful chemicals. This radiation does, however, damage DNA and is responsible for sunburn.
|
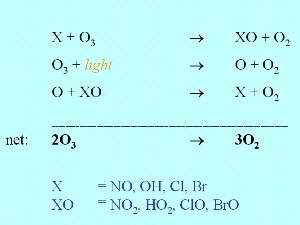
Ozone depletion by radicalsMeasured concentrations of ozone cannot be simply explained by the Chapman reactions. Scientific research conducted since 1970, has shown that nitrogen oxides and reactive halogen radicals are also involved in ozone chemistry. The scientists, Paul Crutzen, Mario Molina and F. Sherwood Rowland, won the 1995 Nobel Prize for Chemistry for their work showing how chlorofluorocarbons destroy ozone.
|
|
|
Ozone is not only removed by ultra-violet radiation from the Sun but also by reaction with reactive compounds such as nitrous oxide (NO), the hydroxy radical OH or halogen radicals such as Cl and Br. A slight decrease in ozone concentrations was predicted by scientists once significant amounts of halogen containing species started to be released into the atmosphere by human activity. However, no-one predicted that such a dramatic decrease in ozone levels in the stratosphere would occur over Antarctica.
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
|