> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - The big misunderstanding
> English > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - The big misunderstanding
|
Upper AtmosphereRead more |
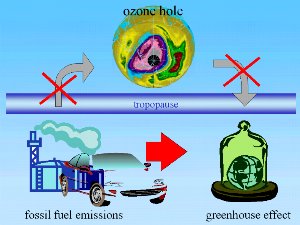
The ozone hole and global warmingIf environmental problems are discussed, people tend to link the ozone hole and global warming together. The fact is that the ozone hole is not a direct consequence of global warming and global warming is not a direct consequence of the ozone hole. But since nearly everything in the climate system is related there are, however, links between the two effects.
|
Where are these links?Global warming is a phenomenon that mainly affects human life and causes the troposphere to warm. The ozone hole forms in the stratosphere. By reducing the amount of ozone at altitudes of between 15 and 40 km, the ozone hole allows more harmful ultra-violet radiation to reach the Earth.
|
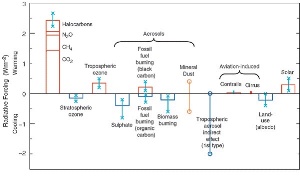
Chlorofluorocarbons (CFC's) play a role in both global warming and ozone hole formation. In the troposphere, they act as greenhouse gases. They absorb infra-red radiation coming from the surface of the Earth and, by trapping this heat close to the Earth they contribute to global warming. In the stratosphere they are broken down by high intensity ultra-violet radiation from the Sun into chlorine radicals and these have the ability destroy ozone. Other greenhouse gases, such as carbon dioxide and methane, do not have a comparable role in ozone depletion.
|
|
|
Since ozone prevents high intensity ultra-violet radiation from reaching the surface of the Earth and causes stratospheric warming, it can be assumed that formation of the ozone hole changes the total radiation budget of the Earth. This is, indeed, the case. However, ozone depletion and the formation of the polar ozone holes doesn't lead to a further warming of the troposphere, but to a slight cooling.
|
|
The impact of the ozone hole on the Earth's radiation budgetIt is not directly obvious that the ozone hole should cause so called 'negative radiative forcing', i.e. lead to a cooling of the troposphere. Instinctively we think, as the ozone layer becomes thinner, more high energy ultra-violet radiation reaches the Earth's surface. This means more energy from the Sun. This is certainly true and is the main reason for the increasing risk of skin cancer. However, there is an effect which counteracts this.
|
|
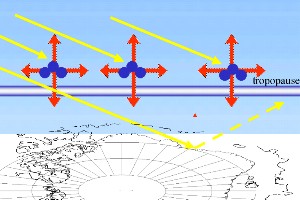
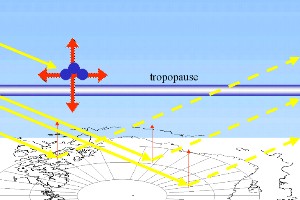
As we know, absorption of ultra-violet radiation by ozone molecules causes warming in the stratosphere. Some of this heat emitted in the stratosphere is transferred to the troposphere causing slight tropospheric warming as well. In addition, in the lower stratosphere, ozone can still act as a greenhouse gas and absorb infra-red radiation coming from the Earth's surface. So absorption of both ultra-violet and infra-red radiation by ozone leads to a warming of the upper troposphere. If ozone levels decrease, the upper troposphere will, therefore, get cooler.
|
|
But there is also an opposite effect. Less absorption of ultra-violet radiation by ozone means more light and, therefore, more energy from the Sun reaches the ground. If this energy is converted to heat, the troposphere should get warmer. However, a proportion of the solar radiation which does enter the troposphere is simply reflected (backscattered) into space and the energy is lost. Backscattering of solar radiation is particularly strong over the Antarctic where the strongest ozone depletion occurs. This is because the snow and ice covered ground has a very high albedo (between 0.6 and 0.8 - which means that between 60% and 80% of the radiation that hits the ground is scattered directly back into space). Because of this high backscattering, only a small fraction of the extra ultra-violet radiation that enters the troposphere from ozone loss causes heating. Overall, the cooling effect of ozone loss is the highest and decreases in ozone levels cause cooling not only in the stratosphere but also slight cooling in the troposphere.
|
The impact of global warming on the ozone holeThe next section on stratospheric cooling shows how global warming in the troposphere causes stratospheric cooling. This cooling promotes ozone depletion in the Southern Hemisphere and perhaps also in the Northern Hemisphere.
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
|