> ACCENT en > UQ 1 Nov Dec 06 Particles in air > F: Polarity and ions
|
|
 |
Fundamentals B2: Polarity and Ions
 Bonds in salts Bonds in salts
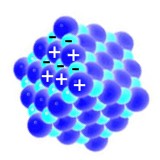
Water forms easier a liquid or a solid since the smallest particles of water attract each other. We say: Water molecules are polar. They behave like little magnets, which stick to each other.
|
For salts it is even easier than for water to turn into a solid state. Their smallest particles, the positive and negative ions, attract each other heavily.
Just ask your parents for an old pot, which is not needed anymore. Put it on the stove and give a teaspoon of dry table salt into it. Switch the stove on and choose the highest grade. What happens?
|
 |
 |
 |
|
1. Polar hydrogen bonds in liquid water; image: Elmar Uherek
|
|
|
As you see the enormous heat does not affect the salt. Salts dilute in water but in a dry environment they form stable crystals. This is because their smallest particles, the ions, are strongly attached to each other.
|
 |
 |
|
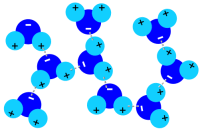
4. Ammonia sulphate strongly enlarged
Source: PSI
|
|
 |
 Salts in the air Salts in the air
We can imagine that the ions in a salt crystal have strong positive and negative charges, which are hard to separate from each other. They attract each other like very strong magnets. If salts are brought into the air or if they are formed in air thanks to a chemical reaction they do not evaporate anymore.
|
|
They form floating salt particles that can attract the humidity of the air. With increasing size they tend to sink to the ground. In order to produce very fine particles a chemical reaction is used, which is also an important source of particles in nature: Sulfuric acid and ammonia form ammonium sulfate salts.
|
 > ACCENT en > UQ 1 Nov Dec 06 Particles in air > F: Polarity and ions
> ACCENT en > UQ 1 Nov Dec 06 Particles in air > F: Polarity and ions