> English > Climate Encyclopaedia > Upper Atmosphere > basics > 1. Understanding the stratosphere > - observation
> English > Climate Encyclopaedia > Upper Atmosphere > basics > 1. Understanding the stratosphere > - observation
|
Upper AtmosphereBasics |
Measurements in the StratosphereThe stratosphere begins at an altitude of between 8 and 15 km and the interesting regions are higher than normal planes can fly. So how do we know about the chemistry of the stratosphere?
|
|
In order to study the chemistry of the stratosphere we can either:
|
AeroplanesUnique measurements have been made possible with special aeroplanes, such as the former Russian high altitude spy plane. This plane, now called "Geophysica", has been converted into an airborne laboratory and such planes can reach altitudes of around 20 km. The flights are very expensive and, as a result, this method is not used often.
|
|
BalloonsA more common alternative is to take measurements using meteorological balloons. Weather balloons can reach altitudes of between 30 and 35 km before they burst. They carry sensors to measure, for example, ozone and send the information back to Earth via a radio signal. As the balloon travels up through the air it sends continuous information back to Earth. Balloons are, therefore, a very useful and relatively inexpensive way of finding out about the vertical structure of the atmosphere.
|
Interaction of molecules with lightThe way in which different chemicals interact with light is really complicated. In very simple terms, something happens when light and matter interact. The light can be absorbed completely by the compound. It can be reflected or scattered directly back into space or can be taken up and re-emitted at a different energy (as a different wavelength).
|
Its easy to see the impact of light absorption by clouds, water and large particles- direct sunlight is blocked by clouds, as we dive into the sea it becomes darker as more light is lost and a dust storm makes the sun look pale. Smaller molecules do the same. They can also absorb or reflect light, they can scatter the light back to Earth or absorb the light and re-emit less energetic light of a different wavelength. Examples of this are phosphorescence and fluorescence. These effects happen when chemicals take up daylight and emit different energy light which we can see in the dark. The sort of light re-emitted tells us something about the type of chemical and the intensity of the light tells us something about its concentration.
|
|
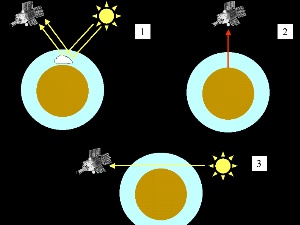
Interaction of light with molecules in the stratosphere can be observed from the ground or measured from space using instruments mounted on satellites. LIDARLidar (LIght Detection And Ranging) is one technique which can be used from the ground. A short pulse of very intensive laser light is sent into the sky. After a while, light returns to Earth and is measured. |
|
|
This light gives us information about the compounds in the atmosphere (from the wavelength of the returning light) and at what concentration they occur (the intensity of the returning light). But how do we know how high up in the atmosphere these compounds are? Light has a certain velocity and the longer the light takes to come back to Earth, the higher the compounds are. The animation on the left shows a laser pulse whose light is scattered back to Earth at different altitudes by air molecules and arrives at the detector at different times. |
|
RADAR and SODARDifferent variations of the wave detection and ranging technique can also be used. The best known is RADAR (RAdio Detection And Ranging), which is used to measure particles in the air and the properties of clouds. RADAR allows us to track thunderstorms over several hundred kilometers. If sound is used instead of light, the technique is known as SODAR (SOund Detection And Ranging) and this gives us a powerful tool for the measurements of wind speed and direction. SatellitesSatellites observe our planet from space. Some of them observe just one area of the Earth and are known as geostationary satellites whereas others orbit the Earth at an altitude of between 500 and 1000 km and can circle the Earth in about 1.5 to 2 hours. Some of these satellites have instruments known as spectrometers aboard and these can detect different wavelengths of light and give us information on the chemical composition of the atmosphere.
|
|
|
|
Related pages To find out more about how light and matter interact have a look at:
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
|