> ENC Master > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - CFC & HCFC
> ENC Master > Climate Encyclopaedia > Upper Atmosphere > more > 2. Ozone > - CFC & HCFC
 |
|
|
|
Higher AtmosphereRead more |
Chlorofluorocarbons and HCFC'sThe investigation of the ozone hole and the measures to ban chlorofluorocarbons (CFC's) world-wide is probably the most important example of a successful environmental protection policy. In this unit we look at the role played by CFC's, what role they are still playing and how they are being replaced.
|
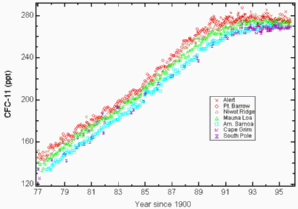
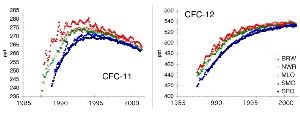
Trends in CFC concentrationsAs seen in Basics Unit 2 the CFCs have been very useful compounds for diverse applications, since they are not toxic and inert gases. For the atmosphere they have a twofold importance. They are greenhouse gases with a very high global warming potential GWP and they have a very long lifetime. The latter allows them to stay in the atmosphere and to be transported slowly to the stratosphere. Although CFCs have been banned within a few years after the Montreal Protocol signed in 1987 it takes a rather long time until they level out. |
Comparison of CFCs and HCFCs
The ozone depletion potential (ODP) is a relative number comparing how dangerous the different compounds are for the ozone layer. CFCs have a twofold negative impact as ozone killers in the stratosphere and as greenhouse gases in the troposphere. The following table gives an overview of a few CFCs compared to the Hydrofluorocarbons HCFC which shall replace them.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
For an overview of more species please click here.
|
|
It is obvious that the lifetime of HCFCs is much shorter. They are primarily decomposed in the troposphere and the probability that they reach the stratosphere and promote the ozone hole destruction is by far lower. However, the global warming potential of this gases is still high. They can be regarded as a compromise but not as the ideal solution.
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute, Mainz
|