|
|
 |
|
|
|
 |
| |
|
|
 |
Higher Atmosphere
Basics |
Chlorofluorocarbons CFC and ozone hole
The story of the ozone hole is a good example of how an apparently harmless class of chemical compounds can become a real danger for life on Earth, and how the governments, industry and civil society can work together to identify this problem and act to resolve it. |
|
|
|
|
 |
|
From this we can learn, that every change man causes in the climate system, may disturb the natural equilibrium in an unexpected way and that if the world community can work together in targetted way, the global environmental problems can be effectively dealt with.
CFCs are just one class among other chemical substances that deplete the ozone layer, but they are the most important one.
|
Use and properties of CFCs
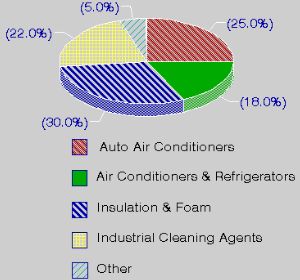
Chlorofluorocarbons are fully halogenated carbon compounds as CFCl3 or CF2Cl2 (commercially they are well known as FREON, although it is a brand name). CFCs have been used in a wide variety of manufacturing steps and products including as a refrigerant, solvent in the electronics industry, foaming or blowing agent, aerosol propellant, fire extinguisher agent, dry cleaning solvent, degreasing agent, a key component in making rigid foam insulation for houses and household appliances, and foam packaging insulation material.
The simple reason for their use is: they do not affect human health in any way because the gases are completely inert. They do not react with any natural compound neither in our body nor in the troposphere. This is why they have an extremely long lifetime and can accumulate in the air. The fatal property of the compounds, we did not take into account, is: They are photolysed by UV light emitted by the Sun.
|
 |
 |
 |
|
1. Use of CFCs
Information from US Environmental Protection Agency (EPA)
|
|
 |
 |
|
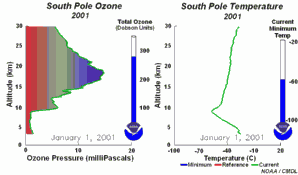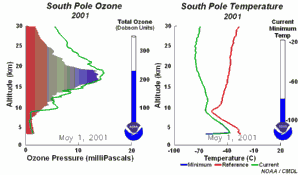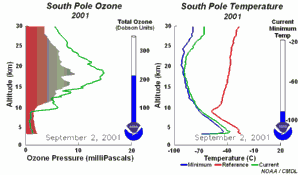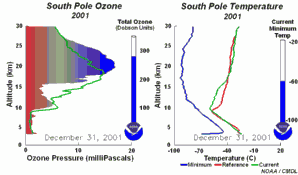
2. The development of the ozone hole 2001
Please click to enlarge a sequence of 5 days (270 K)!
Original animation provided by the NOAA Climate Monitoring and Diagnostics Laboratory, Boulder, Colorado
|
|
 |
The stratospheric fate of CFCs
Since strong UV-B light from Sun is blocked by the ozone layer in the stratosphere, the UV light reaching the troposphere is too weak to degrade the CFCs significantly. But this process did take place as CFCs slowly penetrated the stratosphere. Decomposition leads to the formation of chlorine and fluorine radicals. But this does not necessarily lead to a strong ozone depletion, because the chlorine compounds, first of all responsible for the ozone depletion, undergo also other reactions depending on the meteorological conditions. Although stratospheric ozone is reduced also in other latitudes, the ozone hole is formed only in the polar regions, in particular over Antarctica and only during springtime. What is the reason?
|
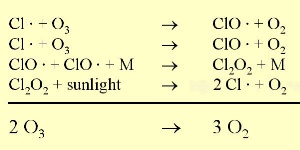
The chemical reaction
Ozone is in a state of equilibrium in the ozone layer, formed and decomposed due to UV-light. Chlorine radicals (Cl ) are catalysts which lead in a chain reaction to the depletion of ozone only. They are very efficient in ozone decomposition, because they are not consumed during this reaction but always recycled. ) are catalysts which lead in a chain reaction to the depletion of ozone only. They are very efficient in ozone decomposition, because they are not consumed during this reaction but always recycled.
|
 |
 |
 |
|
3. Chlorine ozone reaction. Dots indicate, that the reaction partners are radicals.
Full size: 35 K
|
|
The conditions
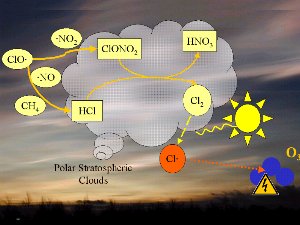
The decomposition of the CFCs leads after further reaction to ClO radicals, which react with nitrogen dioxide NO2 and form ClONO2 or with nitrogen monoxide NO and methane and form HCl and from the reaction ClONO2 with HCl also nictric acid (HNO3). We will not focus on the chemistry in detail but it is important to know, that both products (HCl and ClONO2) do not react with ozone and are rather stable compounds. Only certain conditions lead to reactive chemicals which finally cause the ozone hole. It took two to three years of intensive research after the discovery of the ozone hole in 1985 at the British research station Halley Bey in Antarctica, until this complex conditions have been completely understood. radicals, which react with nitrogen dioxide NO2 and form ClONO2 or with nitrogen monoxide NO and methane and form HCl and from the reaction ClONO2 with HCl also nictric acid (HNO3). We will not focus on the chemistry in detail but it is important to know, that both products (HCl and ClONO2) do not react with ozone and are rather stable compounds. Only certain conditions lead to reactive chemicals which finally cause the ozone hole. It took two to three years of intensive research after the discovery of the ozone hole in 1985 at the British research station Halley Bey in Antarctica, until this complex conditions have been completely understood.
|
 |
 |
|
4. Chemistry on polar stratospheric clouds leads to the dangerous chlorine radicals Cl (red).
Please click to enlarge (100 K)!
|
|
 |
1) One factor is the extremely low stratospheric temperatures of about -80°C or less over Antarctica during the polar night. During such conditions nitric acid and water form stratospheric ice clouds, which are not stable at higher temperatures. On the surface of these ice clouds HCl and ClONO2 react with each other and form nitric acid and pure chlorine Cl2.
2) Chlorine Cl2 is a stable molecule and does not react with ozone but it is easily photolysed by sunlight and forms 2 Cl radicals, which attack the ozone (orange arrow). radicals, which attack the ozone (orange arrow).
|
As we saw above, it is the chlorine radical Cl which leads to the ozone depletion. This does not happen before the sunlight for the cleavage is available, i.e. in Antarctic spring. Therefore we observe the ozone hole every year at the same time in September and October. The reaction chain goes as long as also the other reactants in the ice clouds are thawed again and Cl radicals are removed on other paths. radicals are removed on other paths.
|
 |
 |
 |
|
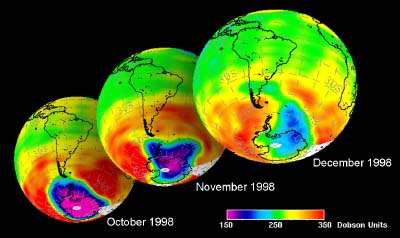
5. Ozone hole development in Antarctic spring 1998
data from GOME
|
|
 |
 |
|
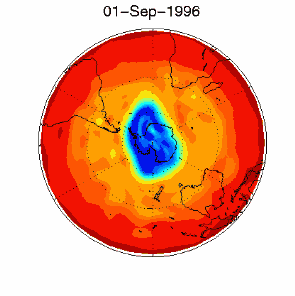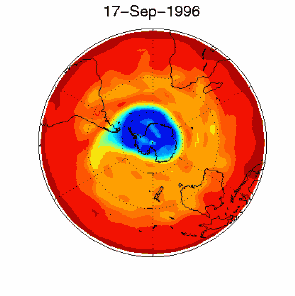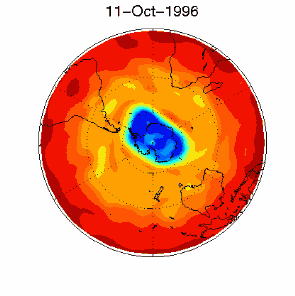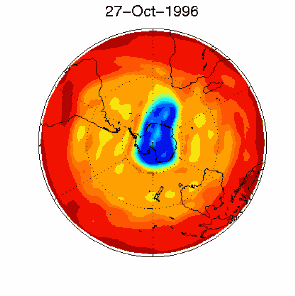
6. The ozone hole in the polar vortex over Antarctica in September / October 1996
adopted from UKMO data set, published at Brown University
Please click for higher resolved version (330 K)!
|
|
 |
3) Finally the whole process should not be that dramatic and not affect the most concentrated part of the polar ozone layer between 14-22 km altitude, because the source compound ClO is usually formed at higher altitudes. A downwind transport in the stratosphere is necessary to transport ClO is usually formed at higher altitudes. A downwind transport in the stratosphere is necessary to transport ClO to lower regions. This takes only place in the so called polar vortex. Here, special meteorological conditions are given in a sort of circumpolar wind around Antarctica. to lower regions. This takes only place in the so called polar vortex. Here, special meteorological conditions are given in a sort of circumpolar wind around Antarctica.
As we see, the conditions for the ozone hole (deep temperatures in polar night, ice cloud formation, polar vortex, followed by the polar sun rise) are that special that this process would have never been predicted by scientists if it would not have been observed.
|
The future of the ozone hole
CFCs are meanwhile banned on a global scale (Montreal Protocol on substances that deplete the Ozone layer-1987 and further amendments). Due to their long lifetime it will take about another 50 years until the CFCs released so far are removed again in the stratosphere and the ozone equilibrium is hopefully stable again. It is assumed that around 2000 the maximum has been reached and the ozone hole during the last years was rather stable in its size. However, exceptions are always possible. In 2002 there has been no significant ozone hole observed. The reason was simple: It was too warm and the polar vortex has not been formed in the usual way. Once again an example that atmospheric processes sometimes ignore any prediction. But in 2003 the hole was back to its former size, the second largest ever observed.
|
 |
 |
 |
|
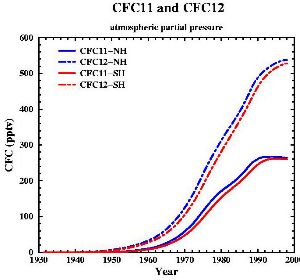
7. Developement of the concentrations of the two most important CFCs (also called FREON 11 and FREON 12).
data from: Walker et al., J. Geophys. Res., 105, 14,285-14,296, 2000 [internet plots]; picture by: Gian-Kasper Plattner (Univ. of Bern, UCLA)
|
|
About this page:
author: Elmar Uherek - Max Planck Institute, Mainz
1. scientific reviewing: Dr. Rajendra Shende, Head Energy and Ozone Action, United Nations Environment Programme 2003-10-06
2. scientific reviewing: Dr. John Crowley - Max Planck Institute for Chemistry, Mainz 2004-05-06
educational reviewing: Hendrik Förster & students, Nordpfalz Gymnasium Kirchheim-Bolanden - March 2004
last published: 2004-06-22
|
|
 |
|









