> ENC Master > Climate Encyclopaedia > Lower Atmosphere > more > 3. Ozone & fire > - fire
> ENC Master > Climate Encyclopaedia > Lower Atmosphere > more > 3. Ozone & fire > - fire
 |
|
|
|
Lower AtmosphereRead more |
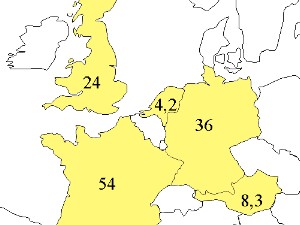
Fire chemistry and global importanceEvery year large areas of our planet burn: 10 Mio ha of boreal forest in the northern latitudes 1 Mio ha = 1 Mio x 100 m x 100 m = 10,000 km2 Imagine the area:
|
|
| |||||||
|
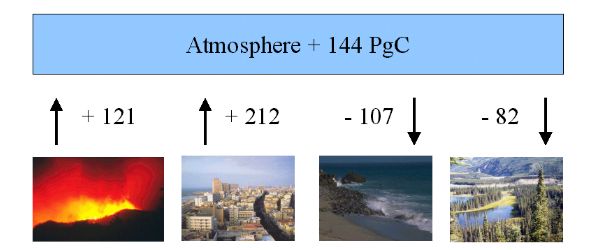
The global carbon budget of CO2 exchange for the period 1850 - 1990 Emissions from man made fires are an important source. |
 |
|
3. Carbon dioxide - sources and sinks - image: Elmar Uherek
|
|
The main sources are:
|
The main sinks are:
|
|
The CO2 uptake by the continents is poorly understood. Probably it is primarily due to increasing plant growth at other places than the typical burning regions. 90% of the CO2 emissions from landuse change are from deforestation. A major problem in the rainforests is, that once burnt down a secondary vegetation grows, which burns yearly. This prevents a reforestation.
|
 |
|
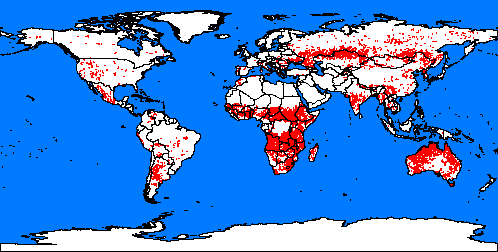
4. Fires spots around the word
|
What is formed during the combustion process? This depends on What do plants consist of? The water content of plants can be up to 60%, but usually before the fire seasons the vegetation looses a lot of water during drought times. A typical composition of the organic dry matter is: elemental: components:
|
|
|
Lignin is formed by the irreversible removal of water from sugars to create aromatic structures. The OH groups can react with each other or with the aldehyde or ketone groups. The result is a polymeric structure with ether bonds. The burning process: After the ignition a period of open fire (sufficient oxygen supplied) is usually followed by a period of smouldering fire (not enough oxygen available).
|
|
| |||||||
|
After ignition (> 180°C / 450 K) flaming combustion (> 580°C / 850 K) starts: Simple molecules as CO2, H2O, NO, N2O, N2 and SO2 are released as oxidation products. After that the flames cease ...
|
... and smouldering (< 580°C / 850 K) begins. Due to a lack of oxygen CO and many partially oxygenated organic compounds (formaldehyde, acetaldehyde, methanol, acetone, methane) are released.
|
|
Related pages Find some examples of fires and released gases at:
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz |