> ENC Master > Climate Encyclopaedia > Lower Atmosphere > basics > 3. Ozone and nitrogen oxides > - NOx
> ENC Master > Climate Encyclopaedia > Lower Atmosphere > basics > 3. Ozone and nitrogen oxides > - NOx
 |
|
|
|
Lower AtmosphereBasics |
Nitrogen oxides - Formation and RelevanceNitrogen oxides play an important role in atmospheric processes. How are they formed, why are they important?
|
 |
|
|
1. Traffic - still an important source of nitrogen oxides. |
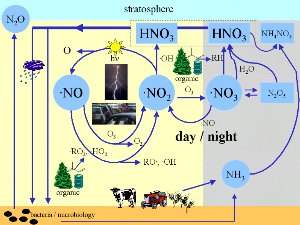
Where do nitrogen oxides come from?The most important nitrogen oxides are nitrogen monoxide NO and nitrogen dioxide NO2. Both together are called NOx. The nitrogen molecules (N2) in the air are very stable and it is not easy to oxidise them. A few bacteria have developed special mechanisms to crack the N-N triple bond and to form oxidised compounds. But by far more relevant are processes where the bonding is cracked by heat. This can only happen under extreme conditions. One example is during the combustion of fuel in a car engine. Most anthropogenic (= human made) NOx comes from this source. It can also happen during other very hot reactions, e.g. in the hottest parts of biomass burning flames. Finally lightning is a major source. In the flash channel temperatures reach up to 30,000 degrees Celsius and easily crack nitrogen bonds. 2. right: Lightning is another important source of nitrogen oxides.
|
|
|
Names of nitrogen compounds:
|
|
Nitrogen oxides as gases are very important for the formation and degradation of tropospheric ozone, because they are involved in catalytic cycles. This is mainly, because NO2 can be photolysed by the sunlight. It forms NO and this NO is oxidised again to NO2. Ozone as well as organic peroxi-radicals (instable oxidised compounds) can be involved in this cycle as we see in detail in the next text.
|
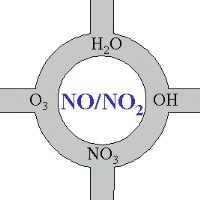
Nitrogen oxides at the crossways of atmospheric chemistryEven if we do not have a closer look on nitrogen oxide chemistry, we can keep in mind, that these compounds are a little bit the heart of atmospheric chemistry. A major part of the chemical compounds, which are oxidised and removed from the atmosphere or transformed into other compounds come into touch directly or indirectly with NO or NO2.
|
|
|
Related pages: Nitrate radicals play a special role at night. Read more about it in:
|
About this page:author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
|