|
|
 |
|
|
|
 |
| |
|
|
 |
Higher Atmosphere
Read more |
Chlorine chemistry
Primarily chlorine chemistry is driving the destruction of the ozone layer. With the industrial production of chlorofluorocarbons (CFCs) man brought a new chlorine source into the atmosphere. Now chlorine has six times the level it had from natural sources with fatal consequences for the ozone layer. However, conditions for the formation of the ozone hole are very special. Therefore a such drastic development has not been predicted.
|
|
|
|
|
 |
 |
 |
|
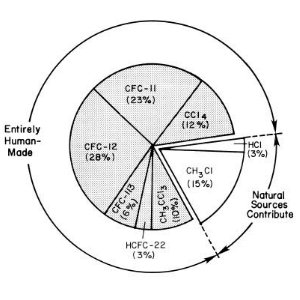
1. Primary Sources of Chlorine Entering the Stratosphere in the Early 1990s
source: UNEP/WMO Scientific Assessment of Ozone Depletion
|
|
 |
CFCs - gases without natural source
The ozone-depleting gases with the largest potential to influence climate are CFC-11 (CFCl3), CFC-12 (CF2Cl2), and CFC-113 (CF2ClCFCl2). It is now clear from measurements in polar firn air that there are no natural sources of these compounds. The only significant natural source of chlorine is methylchloride CH3Cl and this compound has a comparable short lifetime of 1.3 years.
Due to their stability versus the OH radical and photolysis in the troposphere, CFCs have a very long tropospheric lifetime in the range of 50-100 years. They reach the stratosphere and are photolysed, which leads to a formation of chlorine radicals. The availability of these radicals does not necessarily lead to a significant ozone depletion, because other sink reactions compete. Special conditions are needed, as shown in the following sections.
|
The special conditions of the Antarctic ozone hole
During the polar night with temperatures of about -80°C, the very small amount of water available in the stratosphere is able to freeze and to form polar stratospheric ice clouds together with nitric acid (so called nitric acid trihydrate NAT). Now five key conditions can come together:
|
|
|
 |
Second: On the surface of the PSC ice particles the 'reservoir species' for unreactive chlorine, HCl and ClONO2, react with each other to produce Cl2 and HNO3; the latter is immediately incorporated in the particles.
Third: After the return of daylight at the end of the polar night, Cl2 is photolysed to produce 2 Cl radicals. The chlorine becomes reactivated. radicals. The chlorine becomes reactivated.
|
|
|
 |
Fifth: Normally chlorine species as Cl , ClO , ClO , and Cl2O2 are formed and concentrated more in the upper stratosphere and ozone more in the lower stratosphere. Therefore several decades ago experts did not expect ozone to decrease significantly. Ozone and ozone killers should only come together in border zones. At this point, the polar vortex comes into play: Chlorine species are advected to the lower stratosphere by downwind transport from the middle and upper stratosphere within this meteorologically stable vortex (circumpolar wind) with the pole more or less at the center. This is how it comes that the ozone-destroying chlorine species are transported down to lower altitudes, to this region where most of the ozone is accumulated. , and Cl2O2 are formed and concentrated more in the upper stratosphere and ozone more in the lower stratosphere. Therefore several decades ago experts did not expect ozone to decrease significantly. Ozone and ozone killers should only come together in border zones. At this point, the polar vortex comes into play: Chlorine species are advected to the lower stratosphere by downwind transport from the middle and upper stratosphere within this meteorologically stable vortex (circumpolar wind) with the pole more or less at the center. This is how it comes that the ozone-destroying chlorine species are transported down to lower altitudes, to this region where most of the ozone is accumulated.
|
All five conditions have to come together, to form the ozone hole. This is why the major ozone depletion occurs over Antarctica and only in the Antarctic spring months September / October as soon as the sun rises after the polar night. In some years, we have comparable conditions over the Arctic in March and a little ozone hole forms over Northern Europe as well. Later in the year, the polar clouds dissolve, nitrogen oxides become available again, the vortex breaks together, chlorine radicals are removed and the ozone layer recovers.
|
 |
 |
 |
|
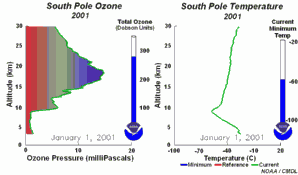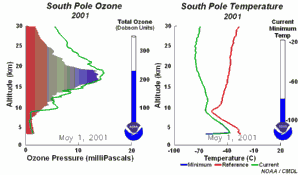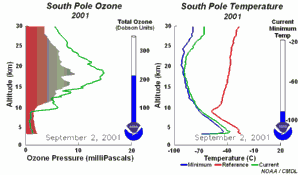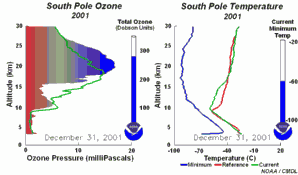
4. The development of the ozone hole 2001
Please click to enlarge a sequence of 5 days (270 K)!
Original animation provided by the NOAA Climate Monitoring and Diagnostics Laboratory, Boulder, Colorado.
|
|
M*: In any sort of reaction A + B -> C a third partner is needed, which takes away excess energy. Otherwise the product C would have the same energy than the sum of the educts A + B and directly react back. In most cases M is the nitrogen from the air N2.
|
About this page:
author: Dr. Elmar Uherek - Max Panck Institute for Chemistry, Mainz
scientific reviewer: Dr. Christoph Brühl, Max Planck Institute for Chemistry, Mainz
educational proofreading: Michael Seesing - Uni Duisburg - 2003-08-07
last published: 2004-05-11
|
|
 |
|









