> ENC Master > Climate in brief > - Clouds, Aerosol
> ENC Master > Climate in brief > - Clouds, Aerosol
 |
|
|
|
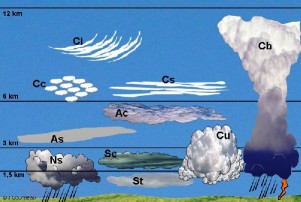
Cloud types and formationBesides from stratospheric ice clouds, which can be seldom observed and usually only in polar regions, all clouds form in the troposphere between the ground and 15 km of altitude. We give latin names to clouds depending on their shape and altitude. Some cloud types lead often to rain, some other like the high clouds hardly ever. Clouds consist of water droplets or little ice particles if the surrounding air is colder than 0°C. Droplets form during a process, which is called condensation. This takes place if the concentration of water molecules, which are in the air as water vapour, becomes too high. We say, the air is saturated with water and cannot keep more moisture.
|
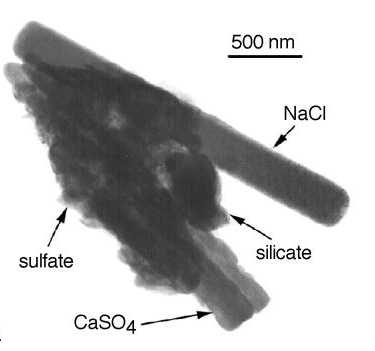
Particles / AerosolsAll liquid or solid particles in the air not consisting of water are called aerosol (matter solved in the air).
|
|
Fungi spores, bacteria, pollen, products of biological degradation ... all this can be called aerosol and some of the particles can be of 100 µm size or even bigger. At the other end of the size range aerosols can also consist of a few molecules, so called molecular clusters. Modern particle measurement allows to detect particles down to 3 nm size (i.e. three millionth of a millimetre). Typical candidates are aerosols of sulphuric acid or small organic aerosol just formed in a chemical reaction in the air itself.
|
|
|
A possible way is dry deposition, i.e. simply the process of descent due to gravitation and sticking to surfaces. Another way is when it rains and particles are washed out, caught by raindrops and brought back to the ground. Aerosols close to the ground (< 1,5 km) remain for half a day up to two days in the air. With increasing altitude the residence time increases, too. Aerosols, catapulted to the stratosphere during a volcano eruption, may remain 1-2 years in the atmosphere. Like clouds also particles have an influence on the light which is passing the atmosphere on its way to Earth or comes back as heat radiation from the Earth. Particles can reduce the transparency of the atmosphere.
|
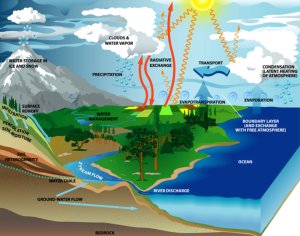
The water cycleCompared to the 1.4 billion km3 of water stored in the oceans, the tiny amount of 12.900 km3 (about 0.001% of the Earth's water resources) in the atmosphere seems to be negligible. However, for the climate system it is important. First, the water in the air is a system in continuous movement. About 500.000 km3 travel every year through the air, evaporate, condense, fall down as rain and snow. The atmospheric amount is 40 times exchanged. Second, only in the atmosphere water has a big impact on the light on its way to the Earth surface or back to the space. And if the amount of water in the air becomes higher due to global warming and we have more clouds in average, this has a strong impact on the energy balance of our planet.
|
|
|
Cloud impact on the climate systemIf clouds are white on top they reflect sunlight like ice and snow. But they also can keep the atmosphere warm like a greenhouse gas due to absorption of heat radiation. Both effects influence the average temperature on Earth, positively or negatively. Our Earth has an average temperature of 15°C. See on the left, what the temperature would be, if all the Earth would be covered by snow, desert, agricultural land and forest or oceans. You can imagine that 10% more clouds white as snow would have a strong influence. However, clouds are not always white and the greenhouse effect of some clouds can outweigh the increased reflection of sunlight (= increased albedo).
|
 |
|
7. Different clouds have different albedos. Author: J. GourdeauAs we see, clouds have very different properties, which in addition depend on the properties of particles in the atmosphere. This makes it very difficult to foresee what happens, if global warming leads to a higher concentration of water vapour in the atmosphere and therefore to the formation of more clouds. Please have a look on the topic CLOUDS & PARTICLES in the Climate Encyclopaedia in order to learn more.
About this page:author: Dr. Elmar Uherek - MPI for chemistry, Mainz
|