|
|
 |
|
The following experiments show you which gases can act as greenhouse gases.
You will need the following materials and chemicals:
|
1 |
dry round-bottomed 1 litre flask made of clear glass |
|
1 |
thermometer marked with 0.1°C units |
|
1 |
stopper to fit the flask with a hole drilled in it for the thermometer |
|
1 |
infra-red light source |
|
1 |
stop watch |
| |
retort stands, clamps, sockets |
| |
carbon dioxide |
| |
methane (natural gas) [F+; R: 12; S: 2-9-16-33] |
| |
water |
| |
air (compressed air or a bicycle tyre-inflator with normal air) |
|
optional |
other gases (e.g. nitrogen, oxygen, etc.) |
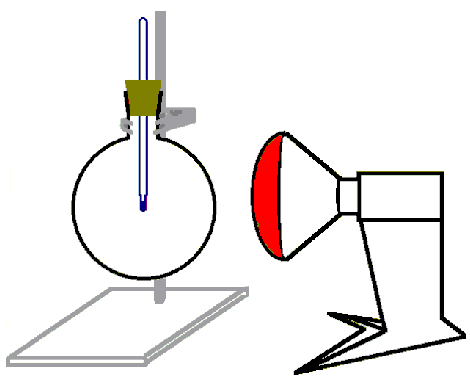
Set up the experiment as shown in Figure 1:
|
 |
 |
|
1. Figure 1
Experimental set-up. The lamp needs to be about 20 cm from the flask for these experiments.
© 2004 M. Seesing, Tausch Universität-Duisburg-Essen, Duisburg.
|
Put the thermometer into the stopper.
Fill the flask with carbon dioxide.
Put the stopper into the flask and make sure that the end of the thermometer is in the middle of the flask.
Illuminate the flask with the infrared-lamp for 5 minutes.
Measure the temperature every 30 seconds.
Repeat the experiment with:
a) air
b) methane [TAKE CARE!! Methane is very flammable and has a lower density than air]
c) air that is saturated with water (add some water to the flask and shake it vigorously just before the experiment)
d) other gases.
Record the temperatures you measure in the table below:
| |
temperature |
|
time [sec] |
carbon dioxide |
air (dry) |
methane |
air (humid) |
. |
. |
|
0 |
. |
. |
. |
. |
. |
. |
|
30 |
. |
. |
. |
. |
. |
. |
|
60 |
. |
. |
. |
. |
. |
. |
|
90 |
. |
. |
. |
. |
. |
. |
|
120 |
. |
. |
. |
. |
. |
. |
|
150 |
. |
. |
. |
. |
. |
. |
|
180 |
. |
. |
. |
. |
. |
. |
|
210 |
. |
. |
. |
. |
. |
. |
|
240 |
. |
. |
. |
. |
. |
.. |
|
270 |
. |
. |
. |
. |
. |
. |
|
300 |
. |
. |
. |
. |
. |
. |
|
Show your results on the temperature-time-diagram. Add your own temperature scale.
|
 |
 |
|
2. time temperature table
|
Arrange the gases according to their ability to absorb heat radiation. Start with the gas that absorbs heat best.
Write the following symbols into the boxes: >> much better than; > better than; ~> only a little better than
Give reasons for the order above.
|
|
Which of the gases you tested seems to be the strongest greenhouse gas? Give the reasons for your choice.
|
|
About this page:
authors: M. Seesing, M. Tausch - Universität Duisburg-Essen, Duisburg, Germany
scientific reviewing: Dr. Pascal Guyon, Max Planck Insitute for Chemistry, Mainz - 2004-05-10
last update: 2004-05-13
|
|
 |
|







