|
|
 |
|
The chemistry of the atmosphere is very complex. Many reactions need light and only occur at particular wavelengths of light. As a result, the atmospheric chemistry of the stratosphere is very different to that in the troposphere. To help us understand what goes on, scientists use simplified models. One of the models used is the Two-Chamber-Photo-Reactor-Model.
|
 |
 |
|
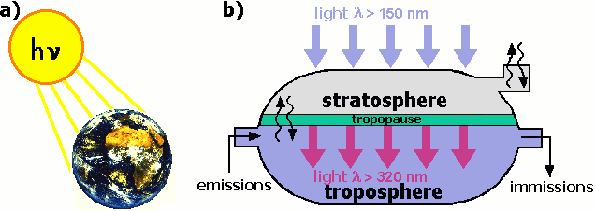
Figure 1
a) radiation: absorption and emission: gamma rays, ultra-violet radiation, visible light, infra-red radiation and radiowaves
b) model of the two-chamber-photoreactor of the atmosphere
© 2004 Seesing, Tausch; Universitšt-Duisburg-Essen, Duisburg
|
|
The absorption spectrum of ozone was elucidated between 1881 and 1890. As early as 1878, Alfred Cornu, a professor of physics, found out that the spectrum of the Sun as it reaches the Earth ends abruptly at wavelengths below 300 nm as if it was cut along a sharp edge. He found that this edge moved to even greater wavelengths when the Sun was low (Figure 1a.).
|
T 1 |
Is this because of the properties of the Sun or the properties of the Earth's atmosphere?
a) How do you think Cornu would have answered this question?
b) Discuss whether you think this is still the correct answer or have scientific developments in the past 130 years changed our understanding? |
|
 |
 |
|
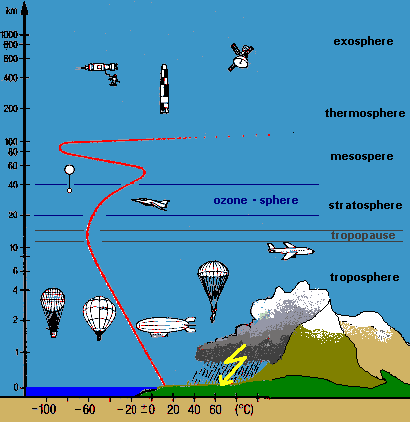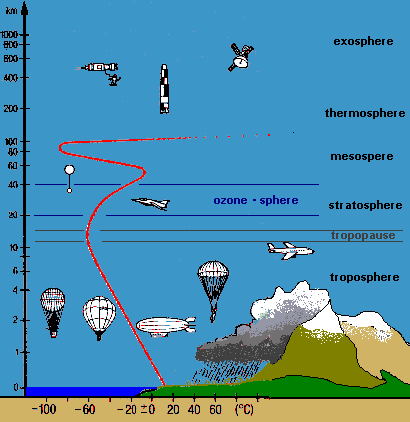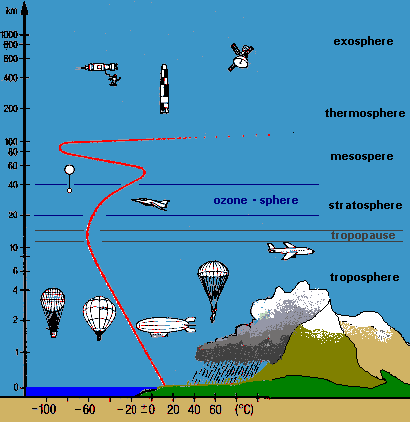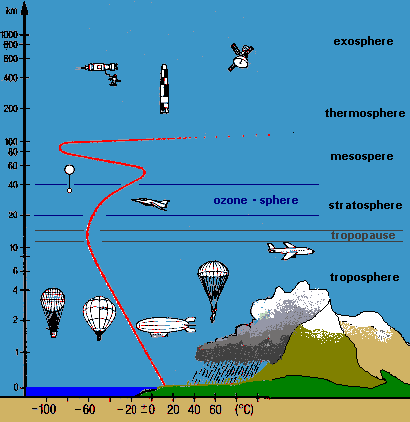
Figure 2:
Layers of the atmosphere and temperature profile
© 2004 Seesing, Tausch; Universitšt-Duisburg-Essen, Duisburg
Please click to enlarge!
|
|
T2 |
Why can we think of the Earth's atmosphere as a two-chamber photoreactor (Figure 1b.)? Use the temperature profile from Figure 2. to help you answer this question. |
|
T 3 |
Why is the temperature in the upper atmosphere much higher than in the tropopause? Use the reactions on the ozone wanted poster in your answer! |
|
T 4 |
Explain why the wavelengths of light are quite different in the stratosphere compared to the troposphere (see Figure 2.). Use the absorption curve from the ozone wanted poster in your answer! |
|
About this page:
authors: M. Seesing, M. Tausch - Universitšt Duisburg-Essen, Duisburg, Germany
last update: 2004-05-13
|
|
 |
|







