|
|
 |
|
|
|
 |
| |
|
|
 |
Clouds & Particles
More |
Properties of particles
Atmospheric aerosol particles have many different sources. Once in the air they undergo lots of different reactions which change their properties. This means that aerosols have a wide range of chemical and physical characteristics and these control how they behave in the atmosphere.
One of the reasons why aerosols are so important is because they are essential for the formation of clouds. |
|
|
|
|
 |
The chemical composition of aerosols
The chemical composition of particulate matter is strongly related to its origin. The major chemical components of aerosols are sea-salt, sulphate, nitrate, ammonium, organic material, crustal species, trace metals and water.
The largest aerosols (which have a diameter greater than 1 µm) are known as coarse mode aerosols. These particles consist of chemicals found in the Earth's crust (such as silicon, aluminium, iron and calcium), those found in sea-spray (primarily sodium and chloride), biological elements (pollen, spores, insect debris) and coal fly ash.
Particles with diameters smaller than 1 µm are known as fine mode aerosols. These generally form either as a result of secondary reactions in the atmosphere such as gas to particle conversion (for example, nitrate, sulphate and some organic carbon compounds) or are emitted during high temperature combustion processes (such as lead, zinc and nickel and elemental carbon).
|
Chemical mixing
As the atmosphere is continuously moving and changing, the chemical composition of a particle often alters during its atmospheric residence time, which is typically a few days. Two mixing states occur: internal and external mixtures.
|
|
In an external mixture, particles from different sources remain separated, i.e. not attached to each other. |
 |
In an internal mixture, the various chemical components are mixed within a single particle. The older the air mass is, the greater the degree of internal mixing.
|
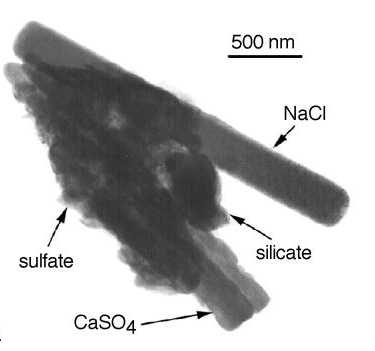 |
 |
|
1. TEM image of mineral dust collected from the marine troposphere. Copyright © 1999, The National Academy of Sciences
|
Cloud Condensation Nuclei
Aerosols are essential for the formation of clouds, providing a site for water vapour to condense onto. However, not all aerosols can serve as a nucleus for water drop formation. Those which can are called Cloud Condensation Nuclei (CCN). This ability depends on size, chemical composition and supersaturation (see formation processes).
About half the aerosol particles over the oceans can act as CCN, whereas only 1% of the aerosols in polluted environments can. However, the total concentration of aerosol particles in polluted areas is much higher than over the oceans. CCN concentrations of around 100 per cm3 are typically found in marine air masses whereas concentrations of many thousands of CCN per cm3 are found in polluted air.
To act as a CCN, particles must be hygroscopic, i.e. they must contain sufficient amounts of water-soluble material. This is why the chemical composition of aerosols affects cloud droplet formation. For example, soil dust particles are not very soluble and can't act as CCN, whereas sea-salt particles are efficient CCN (on humid days, it can be difficult to pour salt from a salt shaker because water vapor has condensed on the salt crystals, sticking them together). |
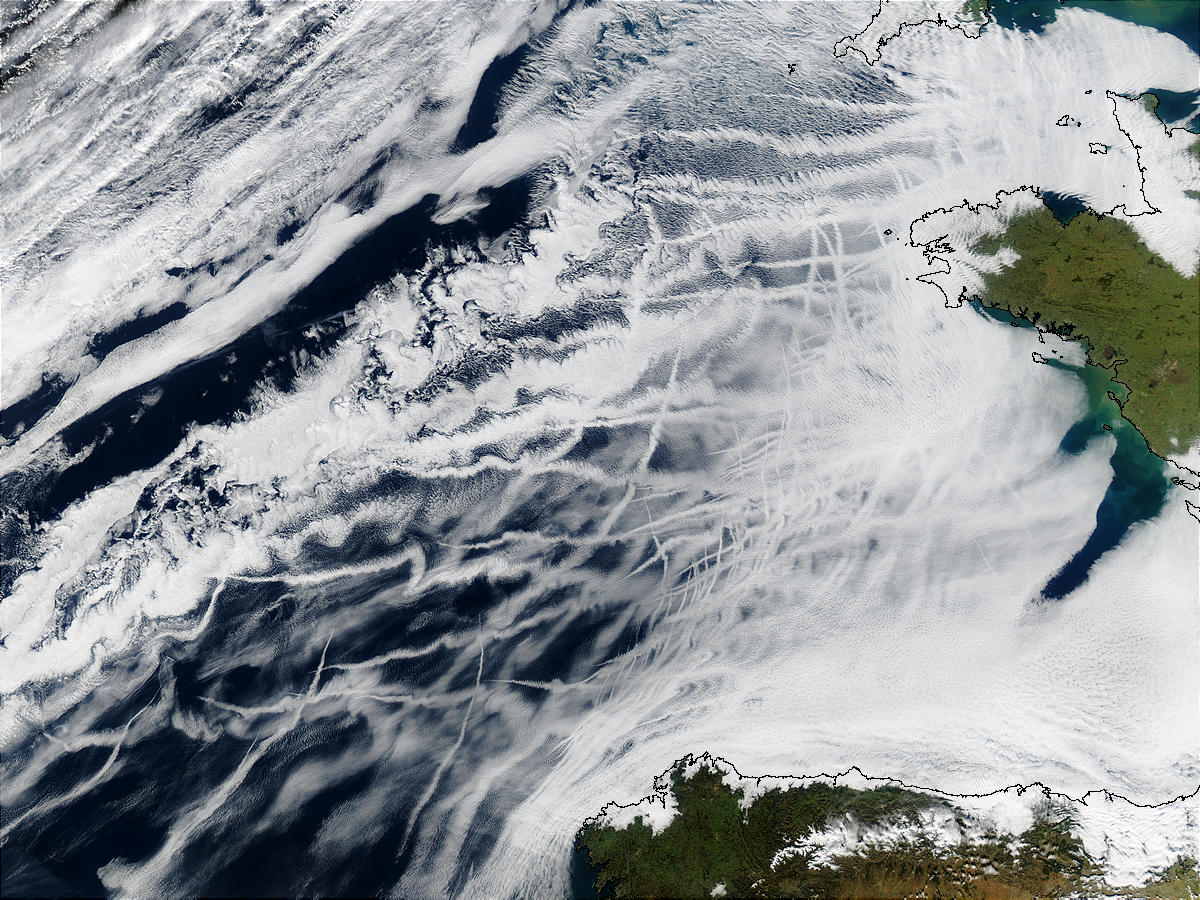 |
 |
|
2. Ship tracks: particles emitted from the exhausts of large ships act as CCN and form the clouds which are shown in this image. France is on the right and Spain at the bottom. As the ships move about the East Atlantic, these clouds form and leave a visible record of where the ships have recently been. Ship tracks can last for hours and give clues to the relative speed of the ships. The faster the ship, the narrower and longer the ship track will be. Slower ships leave shorter and wider ship tracks. Source: NASA.
|
Effects of aerosols on clouds
Aerosol particles are necessary for cloud formation and the size and number of particles changes the characteristics of the clouds. In fact, aerosols are essential players in the cloud system; they change the microphysics of the cloud (the number and size of the water droplets).
One of the fundamental observations is that increasing the number of particles in the atmosphere on which cloud droplets can form, leads to clouds with more, but smaller, droplets. The number and size of droplets is important in governing the rain potential of the cloud and its optical properties. In climate, the influence of aerosols on clouds is called the indirect effect.
|
Therefore, as anthropogenic activities are an important source of particulate matter, humans modify the number and characteristics of clouds.
The photograph on the left shows condensation trails (also called "contrails") over the Rhône Valley in France. These artificial clouds are formed as a result of aeroplane exhausts and are made of ice crystals. It is estimated that these “artificial clouds” cover 0.1% of the planet's surface.
|
 |
 |
 |
|
3. Condensation trails over the Rhône Valley
Source: NASA.-Please click to enlarge!
|
|
 |
 |
4. a+b) Contrails are often seen in the sky on sunny days. |
|
 |
 |
 |
4. b) Authors: C. Gourbeyre, J. Gourdeau. |
|
About this page...
author: J. Gourdeau - LaMP, Clermont-Ferrand, France
scientific reviewer: Dr Paolo Laj - LaMP, Clemont-Ferrand, France
last published: 2004-04-21 |
|
 |
|







