When phytoplankton are infected, die or are eaten, DMSP is released into seawater where it breaks down to form DMS. Much of the DMS stays in seawater and is consumed by bacteria or converted to other chemical species. A proportion, however, escapes and enters the atmosphere.
As DMS is the breakdown product of a biologically produced chemical compound, DMS emissions occur in the spring, summer and autumn when phytoplankton are growing. Emissions come from both coastal waters and from the open ocean but are larger in regions where particular phytoplankton grow. Lots of DMS is emitted from the north east Atlantic Ocean and from the Bering Sea in the North Pacific Ocean as there are regular blooms of coccolithophores in these areas. Lots of DMS also enters the atmosphere from coastal waters around Europe.
|
 |
 |
 |
|
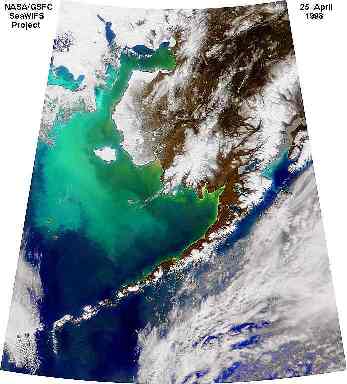
2. The calcium carbonate plates of the coccolithophore Emiliania Huxleyi mean that blooms (large numbers of the organism) are visible in seawater and can be seen by satellites in space. The greeny-turquoise colour is a coccolithophore bloom in the Bering Sea seen from the NASA SeaWiFS Satellite.
|
|







