|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more |
Distribution & concentration (1)
We have already learnt a lot about the gases which exist in our atmosphere. The greatest number of gases are found in the troposphere, the lowest layer of the atmosphere, but their concentrations vary greatly over both time and location.
|
|
|
|
|
 |
How to describe a gas in the atmosphere?
Amount
A gas in the atmosphere can be:
a) a major component of the air (oxygen, nitrogen, argon)
b) a major trace gas (carbon dioxide, methane, ozone, nitrogen dioxide)
c) a minor trace gas (organic gases such as butane, ethanol, CFCs)
Trace gases are gases which make up only a tiny fraction of the air. Levels of these trace gases can be as low as one molecule in one billion or even one trillion air molecules.
|
 |
|
Can you imagine one ppb?
ppb = parts per billion (American *)
more correctly: nmol/mol
1 molecule in 1,000,000,000 molecules
1 Indian in all India, 1 cent in 10 million EUROs, 1 second in 32 years.
And 1 ppt?
ppt = parts per trillion
more correctly: pmol/mol
1 molecule among
1,000,000,000,000 molecules
1 stamp in an area the size of Paris ...
These levels are so small, its really difficult to image them, but modern scientific techniques can detect them.
| | |
The misunderstanding of mixing ratio 'units' ppm and ppb
In numerous scientific publication the amount of a compound in the air is given in ppm (parts per million) or ppb (parts per billion). We also use this abbreviation here, because it is so common and you find it everywhere. But it can be very misleading.
Three typical mistakes:
1) You often see written: The concentration of CO2 in the air is 385 ppm. This is wrong. 385 ppm is a mixing ratio and not a concentration. Mixing ratios give the number of molecules of a certain chemical in a fixed number of air molecules. Concentrations are, for example, the number of moles of a compound in a fixed volume of air, for example 20 nmol m-3 . They have a real unit.
2) Mixing ratios do not really have a unit, because you can cancel the units. If the mixing ratio is 385 ppm this means you have 385 molecules among 1,000,000 molecules. The more correct expression is 385 µmol/mol.
3) The terms billion and trillion are defined in different ways in different countries:
1 American billion = 1 British milliard (seldom used) = 1,000,000,000
1 American trillion = 1 British billion (seldom used) = 1,000,000,000,000
In many other languages, terms related to 1 British milliard (French: milliard, German: Milliarde) are more common than the American billion for 109. So take care!
|
often used: |
more correctly: |
this means: |
|
ppm (parts per million) |
µmol / mol = 10-6
(micromol / mol) |
1 in 1,000,000 |
|
ppb (parts per billion) American |
nmol / mol = 10-9
(nanomol / mol) |
1 in 1,000,000,000 |
|
ppt (parts per trillion) American |
pmol / mol = 10-12
(pikomol / mol) |
1 in 1,000,000,000,000 |
|
Distribution
Depending on the local and temporary conditions in the atmosphere, a gas can be:
|
 |
 |
|
1. a) homogeneously distributed
(e.g. nitrogen, oxygen, carbon dioxide)
image: Elmar Uherek
|
|
 |
 |
 |
|
b) inhomogeneously distributed
(e.g. water vapour, ozone, many minor trace gases)
|
|
|
If a gas is homogeneously distributed this means that we find comparable mixing ratios of the gas everywhere around the globe and also at varying altitudes. This is the case for stable gases with a long life times. These gases are only slowly removed from the atmosphere and are either unreactive or react only very slowly.
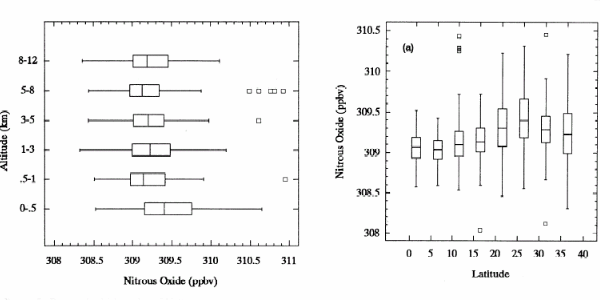
Example: Nitrous oxide N2O |
 |
 |
|
2. Distribution of nitrous oxide in the atmosphere. The graphs show mixing ratios over the Pacific Ocean at different altitudes and latitudes with associated error bars. J.E. Collins et al., Journal of Geophysical Research, 101, D1 (1996) p. 1975-84.
|
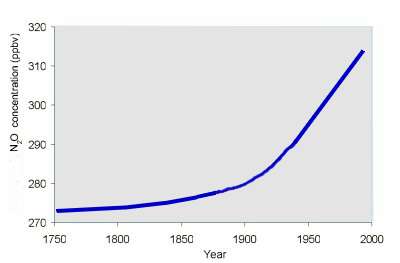
Nitrous oxide N2O is a homogeneously distributed gas, similar amounts are seen all over the world and at different heights in the atmosphere. However, concentrations have increased over the past 200 years as a result, primarily, of human activity.
|
 |
 |
 |
|
3. The change in N2O levels over time. author Elmar Uherek.
|
|
 |
 |
|
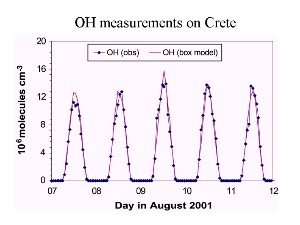
4. Time profile of OH concentrations. Source: Presentation J. Lelieveld, MPI Mainz 2003.
|
|
 |
Development over time
The amounts of some gases depend strongly on the strength of the Sun. The levels of these gases in the atmosphere shows a daily cycle and sometimes also a seasonal pattern
Daily variation: for example the hydroxyl radical OH
The hydroxyl radical is formed from the breakdown of ozone by sunshine. So atmospheric OH levels increase as the Sun rises, reaching a maximum after noon and then decrease through the afternoon and are negliable at night (see the unit on oxidation for more information).
|
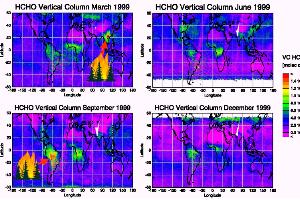
Seasonal variation: for example formaldehyde
Vegetation fires are one source of the gas formaldehyde (HCHO). Concentrations of formaldehyde, as measured by the satellite based GOME instrument, are high during the fires seasons (March in Southeast-Asia, September in Brazil).
|
 |
 |
 |
|
5. Formaldehyde - total columns observed by GOME from space. © IUP Bremen / ESA - GOME. Please click to enlarge (110 K).
|
|
|
Some gases have increased or decreased in the long term over decades, hundreds, thousands or even millions of years. Since the industrial revolution, some 200 years ago, human activity has increased the average concentrations of many gases in the atmosphere.
|
 |
 |
|
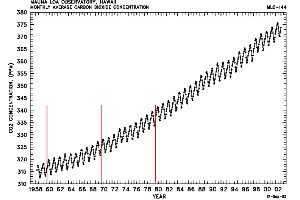
6. The CO2 concentration trend recorded at the Mauna Loa Observatory, Hawaii. graph: © CDIAC US Department of Energy. Please click for a full view! (70 K).
|
|
 |
The growing season and the impact of humans: for example carbon dioxide (CO2)
Carbon dioxide is an excellent example to study the global distribution of a gas. It is rather stable and therefore distributes itself over the whole globe. Atmospheric carbon dioxide levels are continuously increasing because of human activity. Most of the carbon dioxide is emitted from the Northern Hemisphere. The Northern Hemisphere contains much more land, a much higher human population (think about Europe, the U.S.A., China and India) and therefore has a much higher energy consumption than the Southern Hemisphere.
|
 |
 |
|
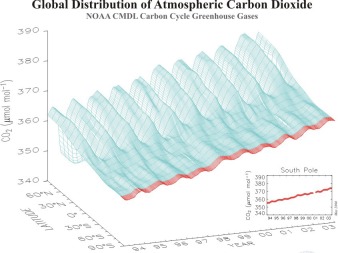
7. Global CO2 distribution and annual and mid-term variations. © NOAA / CMDL. Please click to enlarge! (90 K).
|
|
 |
Carbon dioxide levels, therefore, increase first of all in the Northern Hemisphere and then slowly rise in the south. Transport over the equator takes time since mixing within one hemisphere is much faster than mixing between the hemispheres. Along with this long time period change in CO2 levels we also see that the annual pattern of CO2 varies. In winter, the trees and other plants stop growing and, as a result, take up less CO2. At the same time, we humans heat our houses and emit more CO2. Consequently we see the highest CO2 concentrations at the end of the heating period in May and about 5 ppm less CO2 after the end of the growing period in October. The two graphs clearly show both patterns.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
scientific reviewer: Dr. Rolf Sander - Max Planck Institute for Chemistry, Mainz - 2004-05-18
last published: 2004-05-24
|
|
 |
|







