|
|
 |
|
|
|
 |
| |
|
|
 |
Upper Atmosphere
Read more |
1. Dynamics and aviation - more
Worksheet 2:
Transport, decomposition and the effect of CFC's in the stratosphere
|
|
|
|
|
 |
|
T1 |
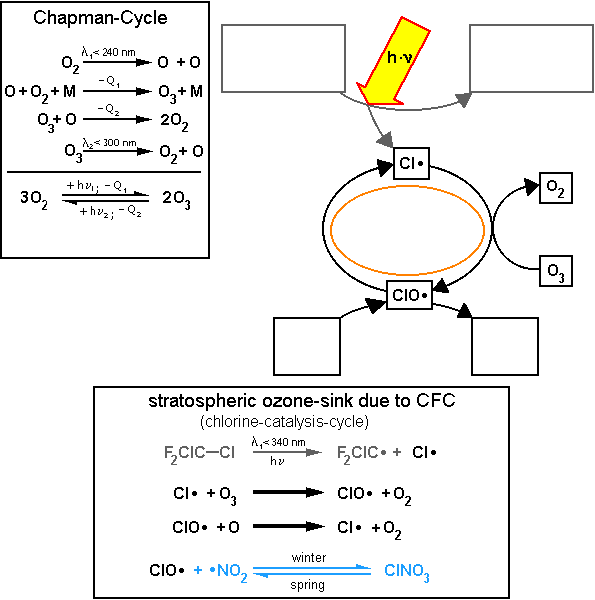
Figure 1. shows the Chapman-Cycle (which describes ozone formation and destruction in the stratosphere) and the chlorine-catalysis-cycle (CCC) depicted as a series of reaction steps and as a reaction cycle. The bold reaction arrows indicate that these elemental processes take place more often than the others (chain reaction). Write the missing formulas in the four squares on the right-hand side of Figure 1. and the name of the reaction process in the empty oval shape. |
|
 |
 |
|
Figure 1: Chapman-cycle and chlorine-catalysis-cycle of stratospheric chemistry (-Q: dissipation of heat)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg
|
|
|
T2 |
The CCC and the Chapman-cycle, which describes the "natural" equilibrium of ozone in the stratosphere, are linked to each other.
Mark where these links occur in red in the reaction cycle in Figure1. and in the box describing the Chapman-Cycle. |
|
T3 |
Show also how nitrogen dioxide acts as "preserving agent" for the chlorine oxide radicals. |
The text has shown you how the CFC's affect ozone levels. The CCC cycles around about a thousand times before the chlorine radicals react with other species or are transported out of the ozone layer. How do the chlorine radicals, which come predominantly from the CFC's, reach the ozone?
|
 |
 |
|
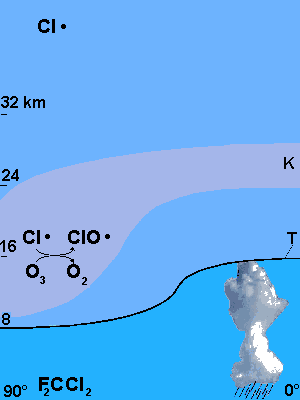
Figure 2: Layers of the atmosphere from the equator to the north pole (T: tropopause; K: area of the stratosphere with high concentration of ozone (>16 DU / km)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg
|
|
 |
|
T4 |
Draw the main air flows in the stratosphere in Figure 2. and name them. |
|
T5 |
Mark the place in the tropopause line where there seems to be the smallest difference in temperature and where an exchange of matter seems most probable. |
|
T6 |
Write a reaction scheme for the photolysis of the CFC's (use F2CCl2 as an example). Show on Figure 2. where the reaction is most likely to take place. |
|
T7 |
Now draw the imagined path of chlorine radicals from the CFC source (using F2CCl2 as an example) to where the chlorine-catalysis takes place. | |
|
|
T8 |
Here are some statements on the CFC problem, on transport processes and on ozone. Mark the right statements with a cross. |
|
q |
The lifetime of CFC's is very long. It is long enough for the CFC's to move to the stratosphere even though their transfer across the tropopause is extremely slow. |
|
q |
The lifetime of CFC's is short, but the transport processes in the atmosphere are fast enough so the CFC's can reach the stratosphere. |
|
q |
Chlorine radicals directly split ozone. The product of this reaction reacts with another chemical species which is necessary for ozone formation. As a result, the formation of new ozone molecules is hindered. |
|
q |
Many chlorine radicals are formed in the stratosphere. These then form stable compounds with ozone and this prevents the ozone acting as a ultra-violet radiaton filter. |
|
q |
Since chlorine radicals catalyse the depletion of ozone, only small quantities of CFC's are necessary for a considerable depletion of ozone. |
|
q |
Catalytic depletion of ozone and transport processes are not the only factors needed to explain the seasonal fluctuation of the ozone concentrations in the atmosphere (ozone hole). |
|
About this page:
authors: M. Seesing, M. Tausch - Universitšt Duisburg-Essen, Duisburg, Germany
last update: 2004-05-13
|
|
 |
|







