|
|
 |
|
|
|
 |
| |
|
|
 |
Upper Atmosphere
Basics |
The layers of the atmosphere
The different layers we see in the atmosphere have different physical properties. As the altitude increases, atmospheric pressure decreases. This is because the density of the air decreases - the higher we go, the less air molecules we find in the same volume of space. Temperature, humidity and wind speed also change with altitude.
|
|
|
|
|
 |
 |
 |
|
1. Blue sky above the clouds. source:www.freefoto.com
|
|
 |
If we look up into the sky from the ground we can't see the layers of the atmosphere, we either see a clear blue sky or clouds. However we get an idea that the properties of the atmosphere change with altitude if we travel by aeroplane. Regardless of the weather on the ground, we see blue sky with no clouds above us once we reach an altitude of 10 - 11 km. At this height we are in the tropopause or even the lower stratosphere. There are no clouds this high up simply because there isn't enough water in the air to allow them to form.
|
Why does the temperature change?
Small scale temperature changes are seen in the atmosphere which occur as a result of local changes in conditions, for example, the land cools down and heats up more quickly than the sea.
There are two main reasons why large scale changes in temperature are seen in the atmosphere:
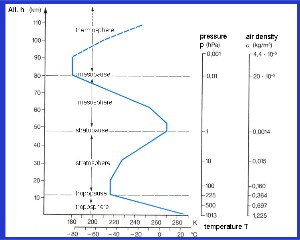
a) the surface of the Earth absorbs sunlight and heats up. As we move away from the warm surface of the Earth, the cooler the air becomes. This leads to a decrease in temperature with altitude.
|
 |
 |
 |
|
2. Profiles of temperature, air pressure and air density with increasing altitude. adapted from: Schirmer - Wetter und Klima - Wie funktioniert das? Please click to enlarge! (120 K)
|
|
|
b) the temperature of the atmosphere is also governed by the chemicals the air contains. Some chemicals are able to absorb sunlight themselves and heat up the air around them. Ozone (O3) molecules in the stratosphere are able to absorb ultra-violet radiation from the Sun and warm the surrounding air. This leads to an increase in the temperature in the stratosphere. The temperature increases with altitude until a local maximum is reached. This temperature maximum defines the border between the stratosphere and the next layer of the atmosphere above. This border is known as the stratopause. The layer above the stratosphere is known as the mesosphere and here temperature decreases with altitude. Another temperature increase takes place in the thermosphere, where nitrogen and oxygen absorb extremely energetic short wavelength ultra-violet radiation from the Sun and are partially converted into charged ions. This layer is, therefore, also known as the ionosphere.
|
 |
 |
|
3. Like a pillow tower:
How air is compressed ...
by Elmar Uherek
|
|
 |
Why does the pressure decrease?
The difference between air and water is that air is compressible and water is not. If you are diving in the sea and have 10 metres of water above you, the pressure is 1 bar, if you have 20 metres of water above you it's 2 bar simply because the amount of water is doubled. However, air is different. Just imagine you have a tower of very light pillows. As the height of the tower increases, the pillows on the bottom of the tower become flatter due to the weight of the ones above. They can be compressed because they have a lot of free space in them. So at the end, you may have 10 pillows in the first 30 cm layer of your tower and only one in the 8th layer even though each pillow weighs the same. This is the same in the atmosphere. Therefore, meteorologists very often use pressure rather than height in metres to define the altitude of the atmosphere. The amount the air compresses depends a bit on the temperature but roughly we can divide the pressure by a factor of 2 for every 5.5 km increase in height.
Click here for more detailed information on how atmospheric pressure is calculated.
Is the thermosphere really that hot?
Temperatures recorded in the thermosphere, 200 - 500 km up in the atmosphere, reach 500 - 1000 oC. Is it really that hot? The problem here is our definition of temperature. In the thermosphere the molecules have a huge amount of energy so the temperatures are correct. However, the number of molecules per volume of space is about one millionth of the number of molecules near the surface of the Earth. This means that the probability that the molecules will collide, transfer their energy and cause heating is extremely low. Therefore, the temperatures recorded in the thermosphere are good measures of molecular energy but not compable to temperatures measured with a thermometer on the ground.
|
4. c) Have a look at the figure on the right and compare the wind speeds at the ground (dark blue, below) and at 9 km altitude (light blue, above) at the same places. What is the wind speed in km h-1 at the three marked locations?
Click the image for full size!
(90 K)
|
 |
|
 |
 |
|
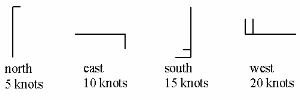
5. Wind speed is often measured in knots where
knot = kn = nautical mile h-1 or in km h-1.
The correct unit is m s-1.
1 m s-1 = 3.6 km h-1
1 knot = 1.852 km h-1
The symbols in the weather map tell us the wind direction (where the wind comes from) and the wind speed in knots. As the example shows, a full sized tick mark represents a wind speed of 10 knots, a half sized tick mark represents a wind speed of 5 knots.
|
|
 |
How does the wind change?
The figure above shows that wind speeds are much greater in the upper troposphere than they are lower in the atmosphere. So a normal wind speed at the tropopause is equivalent to a severe storm at ground level. As a result, air traffic uses a special weather forecasting system to take these changes in wind speed into account. Once we reach the stratosphere, however, wind speed decreases significantly.
|
|
Related pages
Find out more about how the properties of the air change with altitude:
Lower Atmosphere - Basics - Unit 1 - Vertical
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
scientific reviewing: Dr. John Cowley, Max Planck Institute for Chemistry, Mainz - 2004-05-04
educational proofreading: Michael Seesing - Univ. of Duisburg, Germany. Dr. Ellen K. Henriksen - Univ. of Oslo, Norway. Yvonne Schleicher - Univ. of Erlangen-Nürnberg, Germany
last published: 2004-05-05
|
|
 |
|







