|
|
 |
|
|
|
 |
| |
|
|
 |
Clouds & Particles
Basics |
Particles: what do they become?
Once particles are in the atmosphere, they can be transformed, transported and then removed. These processes depend on many factors, including aerosol size, concentration, chemical composition, the location and the meteorological conditions.
|
|
|
|
|
 |
Aerosol concentrations and distributions
The concentration of particles in the atmosphere is the amount of particles per unit volume of air. It can be expressed in terms of the mass of aerosols or the number of aerosol particles per unit volume of air. This means the total mass of aerosols we would find if we weighed all the particles in 1 m3 (1000 litres) of air or the number of particles if we counted them. There are huge differences in aerosol concentrations in different locations. In remote marine areas, the aerosol mass concentration is around 4.8 µg m-3, a factor of 3 lower than concentrations seen in rural continental areas (15 µg m-3). In cities, particle concentrations can exceed 100 µg m-3, that's over 1 million particles in every cubic centimeter of air!
|
 |
 |
|
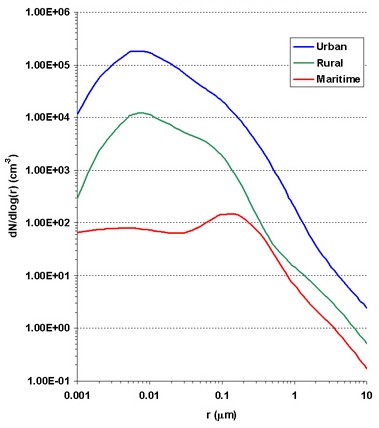
1. Aerosol concentrations for urban, rural and marine environments (Data from Jaenicke, 1993).
|
|
 |
The figure on the left shows the aerosol size distribution for marine, urban and rural environments. Aerosol size distributions show the number of particles in the air as a function of their radius. This figure shows that the highest number of particles are found in cities (the blue line) and that most of these particles are very small, less than 0.05 µm in radius.
The size distribution of urban aerosols is pretty variable. High numbers of small aerosols are found close to their source, but their concentration decreases rapidly with the distance away from their origin.
|
Transformation mechanisms
Aerosols do not stay indefinitely in the atmosphere, they are generally only present in the troposphere for a few days. During their atmospheric lifetime they can be transformed through processes known as coagulation, condensation and by reactions which happen in clouds. These processes are explained in the 'read more' section of this topic.
|
Aerosol removal processes
Aerosols don't stay long in the troposphere. Natural and human sources continuously inject new particles in the air, these particles have to be removed by some process otherwise we couldn't breathe, or see our feet!
The particle removal mechanism is called deposition. Deposition is the word we use to say that the only way for a particle to be removed from the atmosphere is to return to the Earth's surface: it can't disappear by magic! See the 'read more' section of this topic for more details on how aerosols are deposited from the atmosphere to the ground.
|
 |
 |
|
2. This figure shows aerosols being transported in the air. You can see pollution swirling above the Atlantic Ocean off the west coast of France (bottom right). The southern part of the United Kingdom is in the top centre, with Ireland to the west. Source: NASA. Click to enlarge! (68K).
|
|
 |
Of course the bigger the particle is, the shorter length of time it stays in the atmosphere. In the same way a stone falls when you drop it, a large aerosol particle falls under gravity and it falls with a velocity of several centimetres per second. This is why large aerosols are only found close to their origin. However wind can increase the distance a large aerosol travels in the air before it is removed back to the surface. Think about a feather you drop, the feather stays longer in the air if you blow on it! It's the same for particles, even if you can't see them because they are so small. But particles can also travel thousands of kilometers: Saharan dust is sometimes found on the East coast of South America.
|
|
Aparticular case: stratospheric aerosols
The residence time of aerosols in the air (the average length of time a particle stays in the atmosphere before being deposited) is usually less than a week. This isn't always the case for volcanic particles. Large volcanoes erupt and inject material directly in the stratosphere, the upper part of the atmosphere. This material can be primary aerosols such as ash or gases such as sulphur dioxide (SO2). This SO2 can then undergo gas-to-particle-conversion reactions to form secondary aerosols. It's difficult to remove particles from the stratosphere so these particles can stay in the stratosphere for several years and can be spread all over the world.
|
 |
 |
|
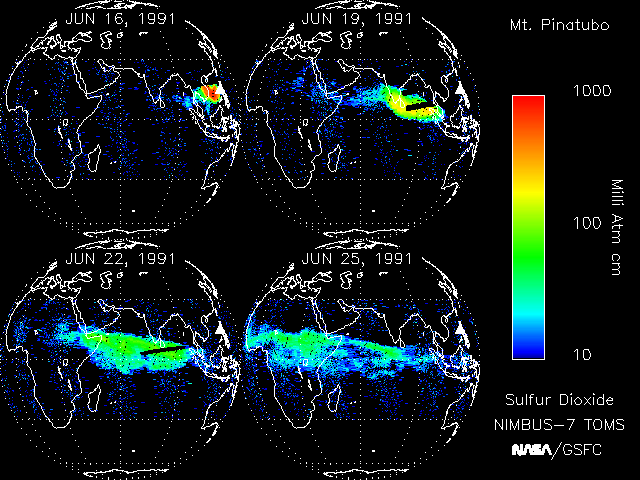
3. In just 10 days, the plume from the Pinatubo volcanic eruption had reached the west coast of Africa. Source: NASA. (Click to enlarge, 32 Kb).
|
|
 |
The Pinatubo eruption
After 600 years of silence, Mount Pinatubo in the Philippines decided to erupt on the 15th June 1991. 20 millions tonnes of sulphur dioxide (SO2) were injected in the stratosphere. In just three weeks, this SO2 had circled the globe (see the image on the left).
|
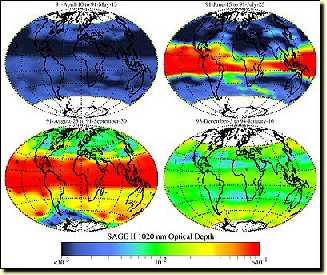
You can also see the "Pinatubo effect" here. The red colour shows the highest values, dark blue the lowest values which are normally observed in the stratosphere.
The first image shows stratospheric aerosol concentrations before the eruption.
The second and third images show aerosol concentrations one and three months after the Pinatubo eruption.
The fourth image shows the aerosol concentrations in the stratosphere two years after the eruption. So even after two years, the atmosphere is still affected by the volcanic eruption!
|
 |
 |
 |
|
4. Aerosol concentrations before and after the eruption.
Source: NASA
|
|
About this page
author: Dr. Justine Gourdeau - LaMP, Clermont-Ferrand, France
scientific reviewer: Dr. Serge Despiau - LEPI, Toulon, France
last published: 2003-09-04
|
|
 |
|







