|
|
 |
|
|
|
 |
| |
|
|
 |
Clouds & Particles
Basics |
Particles: where do they come from?
Aerosols are small solid particles or liquid droplets suspended in the air. They range in size from a few nanometers (that's just 0.000000001 m) to almost 100 micrometers (0.0001 m, the thickness of a hair). Their tiny size means we can't generally see them in the air.
|
|
|
|
|
 |
|
Aerosols come from both from natural and human (anthropogenic) sources. They can be directly emitted as particles (primary aerosols) or they can form as the result of chemical reactions in the air (secondary aerosols).
Sources of primary aerosols
Aerosols can be of natural or anthropogenic origin.
|
Marine aerosols
Aerosols emitted from the sea are known as seasalt aerosols. They are formed from sea spray coming from waves at high wind speeds (this process produces the largest aerosol particles and these aerosols are enriched in sodium chloride), and by the bursting of entrained air bubbles during whitecap formation. Around 1.3 billion tonnes of seasalt aerosol enters the atmosphere each year!
|
 |
 |
 |
|
1. Seasalt aerosols.
Source: Ph. Osset, www.mesvoyages.net.
|
|
 |
 |
|
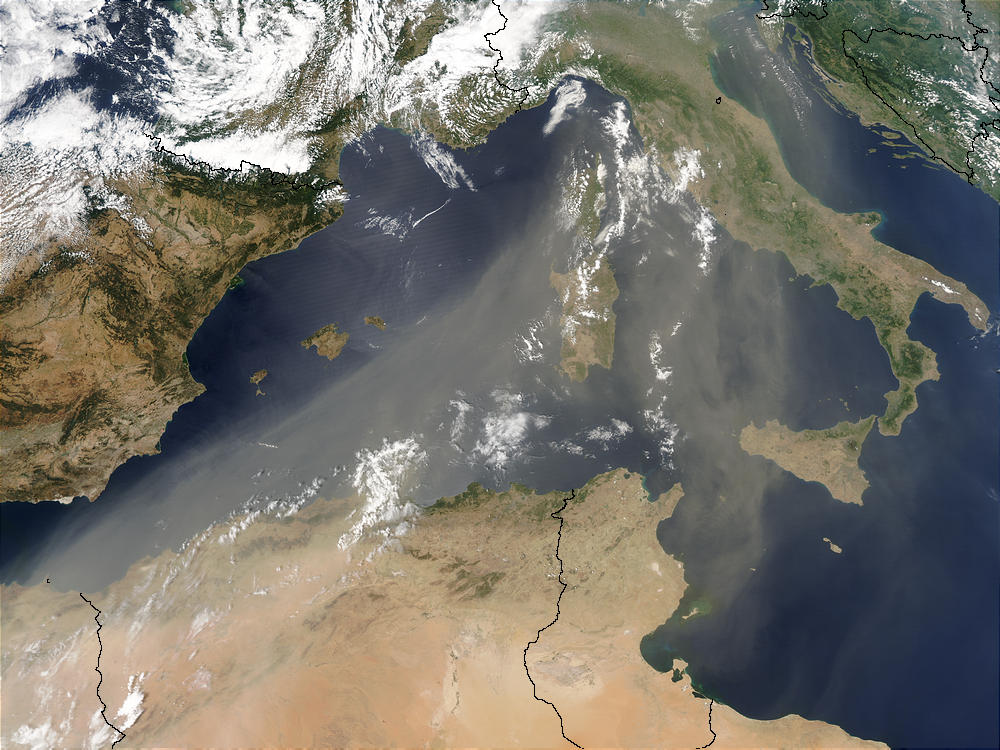
2. A river of Saharan dust flows over the Mediterranean sea and Italy on July 16, 2003.
Source: NASA/Seawifs.
Please click to enlarge!
|
|
 |
Mineral Aerosols
The wind picks up particles from the land surface particularly when the soil is dry and without plant cover and carries them great distances away from their source regions. These mineral aerosols are made up of materials derived from the Earth's crust and are therefore rich in iron and aluminium oxides and calcium carbonate. Most of the mineral aerosols in the air come originally from the desert regions, about half of the total mineral aerosols in the air come originally from the Saharan Desert.
|
|
Primary aerosols emitted into the air as a result of mechanical processes such as wind erosion of soils to produce mineral aerosols or wave breaking to produce seasalt aerosol are large and known as coarse mode aerosols. These aerosols can have diameters of 10 Ám or more).
|
Volcanic aerosols
Volcanic eruptions inject enormous quantities of gases and aerosols in the atmosphere. Unlike the other sources of aerosols, the plume of ash coming from the volcano can be so high that the particles and gases can penetrate the stratosphere. The Pinatubo volcanic plume reached 40 km in height!
Particles that enter the upper atmosphere are not easily removed from the air back to the ground and volcanic material remains at the level injected for a long time (sometimes several years). The gases emitted by volcanoes also produce aerosols. These stratospheric particles have a huge impact on climate and we will look at this in more detail in the 'read more' section of this topic.
|
 |
 |
 |
|
3. St Helens erupting on May 18, 1980. Source: NASA.
|
|
Biogenic aerosols
Particles which are produced by living organisms are called biogenic aerosols. Primary aerosols can be pollens, fungi spores, bacteria and viruses. Forest fires are another source of biogenic aerosols. For example, smoke from forest fires in Malaysia in 1997 resulted in particle pollution 15 times higher than normal over several weeks.
|
Anthropogenic aerosols
Particles emitted during human activity are called anthropogenic aerosols. Anthropogenic aerosols can be either large (coarse particles) or small (fine particles). Dust from roads and construction sites (such as cement works) produces coarse mode anthropogenic aerosols whereas small fine mode aerosols are generated from fossil fuel combustion in power generation and vehicles and from high temperature industrial processes such as metal smelting.
|
 |
 |
 |
|
4. Anthropogenic aerosols.
Source: J. Gourdeau
|
|
|
Many of these aerosol have an impact on our climate, some also have an impact on our health. Particle concentrations are high in indoor air and dust mites, fibres, insect sprays and asbestos are all examples of aerosols which can be very dangerous to human health.
|
5. Scanning electron or transmission electron microscope images. From the left to the right: desertic particle (source: A. Gaudichet, LISA); hibiscus pollen (source:http://uq.edu.au/nanoworld); ash particle from the eruption of Mount St Helens (source:http://volcanoes.usgs.gov); indoor moulds (source:M. Boissier, CSTB); soot particle (MPI for Chemistry Mainz). |
Secondary aerosols
As you've just seen, aerosols can be directly produced from many sources (marine, mineral, volcanic, biogenic, anthropogenic) and in these cases it's a solid material which is emitted into the air. But particles in the air can also result from gas-to-particle-conversion reactions. In this type of reaction new particles can form when molecules gather together in the air to form a species large enough to be considered a particle - this process is known as nucleation. Gases can also condense on pre-existing particles to form bigger aerosols.
Aerosols produced from gas-to-particle conversion reactions are small (less than 1 Ám in diameter) and are known as fine mode aerosols.
|
 |
 |
|
6. Rose. Source: www.freefoto.com
|
|
 |
Plants also emit biogenic gases which we call Volatile Organic Compounds (your nose detects some of these VOC's when you smell a flower). These biogenic gases can under go gas-to-particle-conversion reactions to form secondary aerosols. See the section on plant emissions in the Lower Atmosphere topic for more details.
|
|
Most of the naturally formed secondary aerosols in the atmosphere are the result of sulphur gas emissions. In the marine environment most sulphur is emitted as dimethyl sulphide (DMS) by phytoplankton. Reaction of DMS in the atmosphere forms sulphur dioxide (SO2). On land decaying vegetation and animal material produces hydrogen sulphide (H2S - which smells like rotten eggs...yuk!). Volcanoes also directly release SO2 into the air. SO2 then further reacts to form sulphate aerosols and these are really important to our climate.
Natural sources produce also carbon containing gases which go onto form aerosols.
|
 |
The annual emission of SO2 from human activity has increased from 10 millions tons per year in 1860 to 150 millions tons per year in the 1980's. Anthropogenic emissions of sulphur containing gases now exceed natural emissions even though atmospheric SO2 levels are now falling because of international legislation.
Humans also produce more and more nitrogen containing species that give rise nitrate aerosols. The reaction of some anthropogenic compounds, emitted during combustion of petrol and during biomass burning, results in carbon containing aerosols, which pose a health hazard. |
About this page
author: Dr. Justine Gourdeau - LaMP, Clermont-Ferrand, France
scientific reviewer: Prof. Guy Cautenet - LaMP, Clermont-Ferrand, France
last published: 2004-04-21
|
|
 |
|







