|
|
 |
|
|
|
 |
| |
|
|
 |
Urban Climate
Read more |
The impact of acid rain on human health and the economy
Acid rain has direct and indirect impact on both the natural environment and on people and our infrastructure (buildings, roads, etc.). Deterioration of our historical monuments by acid rain is a particular problem.
|
|
|
|
|
 |
Human health
The pollutants that cause acid rain (mainly sulphur dioxide (SO2) and the nitrogen oxides (NOx)) affect human health, particularly the respiratory system. Have a look in the bioclimate section for more information.
Polluted air
Sulphur dioxide, nitrogen oxides and acid rain can cause respiratory problems including asthma, bronchitis, headaches and eye, nose, and throat irritations. The fine sulphate and nitrate particles which form from the gases are less than 2.5 Ám in diameter (and known as PM 2.5). They can be transported long distances by the wind and then inhaled by people far away from their sources. Because the particles are so small, they get deep into the lungs and can cause cancer.
Reaction of these acid gases with fog can cause acid smog. As a result of pollution control, these acid smogs are now thankfully rare. In 1952, the pH of an acid smog in London was as low as 1.5 and 4000 people died as a result.
|
 |
 |
|
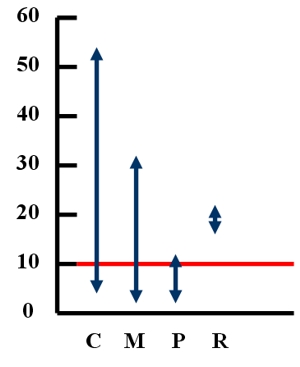
1. Ranges of lead levels in blood, among children, in micrograms per deciliter, in chosen countries. Explanations: C - China (data from 1988), M - Mexico (1995), P - Poland (1992-1994), R - Romania (1995). The red line marks the level of 10 micrograms lead per deciliter of blood. See the text on the left for more information. Author: Anita Bokwa. Source of data: http://www.wri.org/wr-98-99/metals.htm
|
|
 |
Polluted water
Acidic waters corrode water systems and dissolve the heavy metals in pipes so polluting the water. In addition, heavy metals are released into solution when acid rain falls on soils and this water may also enters the drinking water supply. The heavy metals can be absorbed both by plants (through soil and direct contact) and by animals (from food or by direct contact). These metals can then enter the human body where they accumulate causing cancer. Brain damage, kidney problems and Alzheimers have all been linked to people eating "toxic" animals and plants.
Lead levels above ten milligrams per decilitre of blood are known to have substantial negative health impacts. Figure 1. shows concentrations of lead in blood in children from developing countries. The majority of young children in cities in developing countries have average blood lead levels greater than 10 micrograms per deciliter and suffer health problems as a consequence.
|
Buildings and monuments
Acid rain damages stone work in two main ways: dissolution and alterations. It is particularly damaging to buildings made up of limestone and marble. These are primarily composed of the mineral calcite (calcium carbonate, CaCO3) which dissolves readily in weak acid solutions. The reaction which occurs:
CaCO3(s) + 2H+(aq) 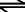 Ca2+(aq) +CO2(g) + H2O Ca2+(aq) +CO2(g) + H2O
converts the solid calcium carbonate into aqueous phase calcium ions and gas phase carbon dioxide and neutralises the acid. As a result, exposed areas of buildings and statues dissolve and carved details are lost. The same process occurs in calcium carbonate rich soils and when soil-dust particles that contain CaCO3 collide with acidified raindrops in the air.
When sulphuric acid covers calcite, the hydrogen ion H+ from the acid dissolves the calcite, as described above. The sulphate ion also released then reacts with the calcium ions and forms a clear-to-white gypsum crust over the marble or limestone:
Ca2+ +SO42- + 2H2O 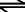 CaSO4 * 2H2O CaSO4 * 2H2O
rain removes some of the gypsum crust over time, creating tiny crevices and causing erosion.
Even though the acidity of rain has been reduced in many places in recent years, buildings are still showing signs of damage. This is because the acid rain has permanently changed the stone from which the building is made. Other materials vulnerable to acid rain damage include carbon-steel, nickel, zinc, copper, paint, some plastics, leather and textiles. Stainless steel and aluminium are more resistant materials.
|
|
The restoration of monuments and buildings is costly. For example, acid rain caused damage to Westminister Abbey in London, England which cost 10 million pounds to repair in the early 1990's. Although economic losses can be calculated, the aesthetic appeal of the world's cultural treasures cannot be price-tagged. The Taj Mahal in India, the Acropolis in Greece and even newer buildings including Canada's Parliament Building and the U.S. Capitol Building are seeing the effects of acid rain.
|
Cars
Automotive coatings are damaged by all forms of acid rain, including dry deposition, and particularly when acid dry deposition is mixed with dew or rain. However, it is difficult to quantify the specific contribution of acid rain to paint finish damage relative to damage caused by other forms of environmental fallout, by the improper application of paint or by deficient paint formulations. Usually the damage is permanent; once it has occurred, the only solution is to respray the car.
|
 |
 |
 |
|
4. Acid rain causes rapid corrosion and rust formation on metal objects used outdoors such as chains, roofs and cars. This,,in turn, generates higher costs of maintenance and renovation. Source of photo: www.freefoto.com
|
|
About this page:
author: Anita Bokwa - Jagiellonian University, Cracow , Poland
educational reviewing: Michael Seesing - University of Duisburg, Duisburg, Germany
last update: 2004-08-05
|
|
 |
|







