|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more |
2. Radiation and greenhouse gases - more
Worksheet 2: A model experiment on the greenhouse effect
|
|
|
|
|
 |
 |
 |
|
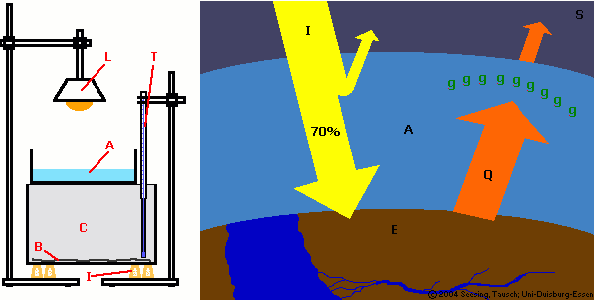
Figure 1: Set-up for
Experiment 1:
L: lamp; T: thermometer
A: water; C: carbon dioxide
B: black cardboard
I: heat insulation (cork plugs)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen.
|
Figure 2: The Greenhouse Effect:
a large part of the sunlight (I) reaching the earth (E) is absorbed, transformed into heat (Q) and the warmer earth re-emits longwave infra-red heat radiation. Greenhouse gases (g) from the atmosphere (A) absorb this re-emitted heat almost entirely and send it partially back to Earth. These gases keep the heat in the lower atmosphere, like in a greenhouse. (S: space)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg.
|
|
|
|
E1 |
Set up the experiment as shown in Figure 1. The experiment consists of a 300 W (tungsten) lamp, a glass container filled with water (1 cm high), a second glass container with black cardboard at the bottom and a temperature sensor in the air space in the lower glass container.
Switch on the lamp, record the temperature every 30 seconds for 5 minutes and plot it on a graph. |
|
T1 |
Carry out the experiment with a) air and b) carbon dioxide in the lower glass container. |
|
T2 |
Repeat both experiments after replacing the black cardboard with aluminium foil. |
| |
temperature |
|
|
background: black cardboard (A1) |
background: aluminium foil (A2) |
|
time [sec] |
air |
carbon dioxide |
air |
carbon dioxide |
|
0 |
|
|
|
|
|
30 |
|
|
|
|
|
60 |
|
|
|
|
|
90 |
|
|
|
|
|
120 |
|
|
|
|
|
150 |
|
|
|
|
|
180 |
|
|
|
|
|
210 |
|
|
|
|
|
240 |
|
|
|
|
|
270 |
|
|
|
|
|
300 |
|
|
|
| |
|
|
T3 |
Write down which part of the experiment E1 simulates which part of the environment by comparing Figures 1 and 2. |
|
The lamp simulates: |
___________________________________________________ |
|
The water in the glass container simulates: |
___________________________________________________ |
|
The air or carbon dioxide in the lower glass container simulates |
___________________________________________________ |
|
The black cardboard on the bottom of the lower glass container simulates: |
___________________________________________________ |
|
T4 |
Experiment E1 is a model experiment simulating the greenhouse effect. Why is it important to put cold water between the lamp and the gas space in the lower glass container? Tick the correct answer. |
The function of the layer of water between the lamp and the gas space is:
c It absorbs the heat coming from the lamp.
c It absorbs some parts of colour from the spectrum of the light of the lamp.
c It prevents the gas from leaking out of the container.
c It simulates the humid clouds in the atmosphere.
|
 |
 |
|
Figure 3: Results from two measuring series.
© Tausch, von Wachtendonk: Chemie 2000+;
Buchner Verlag, Bamberg 2001
|
|
 |
|
T5 |
After about 150 seconds the temperature measured in the experiment using CO2 is about 1°C higher than the temperature obtained using air. Explain this observation using the information in Figure 2: | |
|
|
T6 |
In the model experiment, the greenhouse effect is particularly obvious if there is black cardboard on the bottom of the gas space. You can also get satisfactory results using coloured cardboard, but not using aluminium foil. Why? |
|
T7 |
Greenhouse gases in the lower atmosphere mean that the air next to the Earth's surface is, on average, 33 oC warmer than it would be without these gases. 20.6 oC of this temperature difference is due to the presence of water vapour, 7.2 oC is due to CO2 and 2.4 oC is due to the presence of tropospheric ozone (O3). The air close to the ground contains 0.037% by volume of CO2, but only about 40 ppb O3. How many °C would the contribution of ozone be to the greenhouse effect if its contribution was proportional to its amount in the air? Write down your calculation and result in the space below: |
Compared to CO2 ozone contributes _____________°C to the greenhouse effect.
|
T8 |
In reality the contribution of ozone to the greenhouse effect is much higher than that calculated in T7. Why is this? |
c Ozone is a stronger oxidising agent than the other greenhouse gases.
c Ozone absorbs heat radiation at wavelengths that are not absorbed
by the other greenhouse gases.
c The amount of oxygen in ozone molecules is much higher than in the
other greenhouse gases.
c The ozone close to the ground is formed under strong incident solar
radiation.
|
About this page:
authors: M. Seesing, M. Tausch - Universität Duisburg-Essen, Duisburg, Germany
last update: 2004-05-13
|
|
 |
|







