|
|
 |
|
|
|
 |
| |
|
|
 |
Climate in cities
Basics |
The impact of acid rain on the natural environment
Acid rain affects all elements of the environment, surface waters and groundwaters, soils and vegetation. It negatively affects food chains, reduces biodiversity and damages our world.
|
|
|
|
|
 |
 |
 |
|
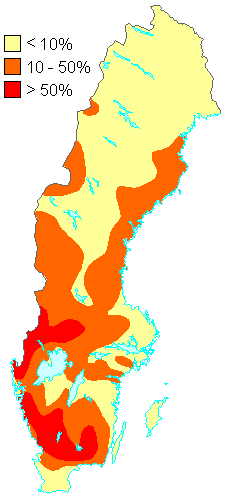
1. Percentage of the total number of lakes in different parts of Sweden judged to be acidified in 1990. The acidification situation has changed little since then. Source: Swedish Environmental Protection Agency.
http://www.internat.environ.se/index.php3?main=/documents/pollutants/kalka/forsure.html
|
|
 |
Acidification of waters
In Scandinavia, acid rain has increased the natural acidity of the lakes and rivers. Some 14,000 Swedish lakes, located in acidic crystalline rocks, have been affected by acidification with widespread damage to plant and animal life as a consequence. This type of damage has also occurred in the United Kingdom, the Alpine region of Europe and North America.
The decrease in European emissions of SO2 and NOx in the 1990's resulted in recovery for some waters. However reduced emissions do not, automatically, translate to an immediate improvement in the water quality in streams lakes and rivers.
|
Soils
When soil becomes acidified essential nutrients such as calcium (Ca) and magnesium (Mg) are leached out before the trees and plants can use them to grow. This reduces the soil's fertility. In addition, acidification may release aluminium from the soil. At high concentrations, aluminium is toxic and damages plant roots. This reduces the plants ability to take up nutrients such as phosphorus, eventually leading to death.
|
The soils most prone to damage are those which overlie acid rocks such as quartz sandstones. Soils that are rich in lime are more resistant to acidity. Soils are less vulnerable to acid rain than surface waters because they have a buffering capacity. This means that the soil can neutralise some or all of the acidity from the acid rain. Unfortunately the buffering capacity of the soil is reduced by continuous deposition of acid rain. In Sweden, in most of the country, the soils are composed of slow-weathering minerals from Scandinavia's Precambrian bedrock. The result is that the maximum amount of acid that the soil is capable of neutralising (the critical acid load) is low. Sweden is, therefore, more sensitive to acid deposition than most other countries.
|
 |
 |
 |
|
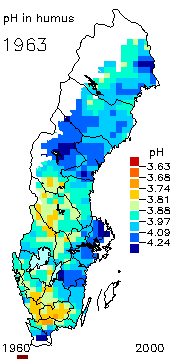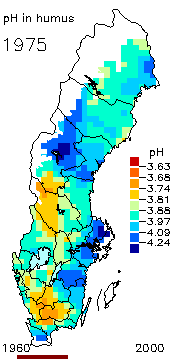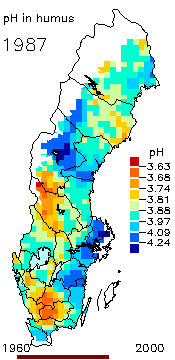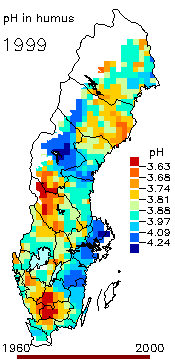
2. The pH level in the humus layer in Sweden. The animation shows changes since 1963. Author: Ake Nilsson, Swedish University of Agricultural Sciences. Source: Swedish Environmental Protection Agency.
http://www.internat.environ.se/index.php3?main=/documents/pollutants/kalka/forsure.html
|
|
Vegetation
Acid precipitation does not usually kill trees directly. It weakens them by educing the amounts of nutrients available to them from the soil, allowing nutrients to be lost from the leaves and exposing them to toxic substances slowly released from the soil. Acidic pollutant gases, like sulphur dioxide, can cause direct harm to plants growing close to large emission sources such as power stations.
|
 |
 |
|
3. Acidity can cause severe damage to trees. Dead forests in the West Karkonosze Range (the Sudety Mountains), at the Polish-Czech border. Photo: Witold Goraczko.
|
|
 |
Acid deposition destroys the surfaces of the leaves and needles of trees and plants. This damage causes uncontrolled water loss and slows photosynthesis. It reduces the rate at which leaf litter decomposes, causes the death of useful microorganisms present in tree roots and reduces the rate at which soil organisms (including bacteria) respire.
|
Forests damaged by acid rain can be found all over Europe and in many regions of Eastern USA. Figures 3. and 4. show damaged forests in the Black Triangle (see the link at the bottom of the page).
|
 |
 |
 |
|
4. Mountain forests are exposed to acid clouds, fogs and rain as well as acidic gases like sulphur dioxide. Dead forests in the West Karkonosze Range (the Sudety Mountains). Photo: Witold Goraczko.
|
|
|
It's difficult to isolate the contribution of acid rain to forest damage from other stresses such as drought, fire and pests. For this reason, the contribution of air pollution to forest damage is a controversial subject, particularly in North America.
|
Food chains and biodiversity
Soil acidification releases metals that can harm microorganisms in the soil as well as birds and mammals higher up in the food chain. The most sensitive groups include fish, lichens, mosses, certain fungi and small aquatic organisms. Some organisms may be completely eliminated, reducing biodiversity. Heavy acidification of soils in the south of Sweden has already brought about substantial changes in biodiversity.
Acid rain also disturbs the natural cycles of sulphur and nitrogen.
|
 |
 |
|
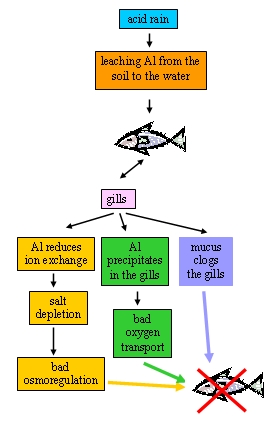
5. Influence of acid rain on fish. See text for explanations!
Author: Anita Bokwa
|
|
 |
Fish and other water-living organisms
When the pH of water is below 5.5, fish die or become seriously ill. Back in the 1950's it was discovered that fish were disappearing from lakes and waterways in southern Scandinavia.
The high levels of aluminum (Al) leached from the soil by acid rain is the cause of fish death. Firstly, aluminium is able to reduce ion exchange through the gills and causes salt depletion. For freshwater fish, maintaining osmoregulation (the ability to maintain a state of balance between salt and minerals in the organism's tissue) is essential to stay alive. Aluminum also precipitates in the gills and interferes with the transport of oxygen, so that fish literally die of suffocation. Secondly, fish will produce mucus to combat the aluminum in their gills. This mucus builds up and clogs the gills so that oxygen and salt transport is inhibited. Fish are then unable to regulate their body salts. Also, a low pH level will throw off the balance of salt in the fish's tissue.
Low pH often stunts the growth of frogs, toads and salamanders. A decline in bottom-dwelling organisms can lead to a decline in the number of species of flies, mosquitoes, craneflies, midges and mayflies. This puts a stress on aquatic carnivores (such as insect-eating fish). Predatory birds can eat the fish and end up with high concentrations of aluminum. The birds will then produce eggs with soft shells and the young rarely survive.
|
About this page:
author: Anita Bokwa - Jagiellonian University - Cracow, Poland
scientific reviewer: Neil Cape - Institute of Terrestrial Ecology, Edinburgh Research Station, Scotland - 2004-08-11
educational reviewing: Michael Seesing - University of Duisburg, Duisburg , Germany
last update: 2004-08-17
|
|
 |
|







