|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Basics |
What does the tropospheric air consist of?
The air is a mixture of a few dominant gases and many many trace gases, some of which are rather important for our climate system.
|
|
|
|
|
 |
 |
 |
|
1. Raising dew in the morning - Virgen valley Osttirol
photo: Elmar Uherek
|
|
 |
The gas phase
The most obvious problem we have with air is, that we cannot see it. But if something is invisible this does not mean, that it does not exist. If you see the dew in the grass in the morning you know that it disappears as soon as solar radiation intensifies. The little water droplets are not taken up by the ground or disappear due to some magic. They simply evaporate and change their state from the liquid phase to the gas phase. This change between a visible and an invisible state is most easily understandable for water. |
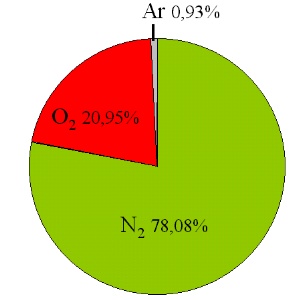
Dry air consists of about 78% nitrogen 21% oxygen and 1% Argon. These gases can also be transferred to the liquid phase. But the temperatures necessary for that are below -150°C and we never observe such processes in nature. Therefore air is a gas and invisible for our eyes.
Particles
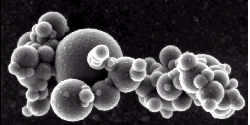
When we see pictures of a sand storm in the Sahara it is very obvious that there is a lot of sand and dust in the air. We can imagine that the same is true, if industrial or car emissions are let to the air in our towns. But even the cleanest air at very remote places, like Antarctica or over the oceans, contains little particles.
|
 |
 |
 |
|
2. Composition of the atmosphere
nitrogen (N2), oxygen (O2) and argon (Ar)
author: Elmar Uherek
|
|
 |
 |
|
3. Aerosol from the Mediterranean Sea
Electron Microscopy Image
Author: Research Group Dr. Helas, MPI Mainz
http://www.mpch-mainz.mpg.de/~kosmo/remgallery/medsea/medsea.htm
|
|
 |
They can be formed by sulphuric acid or water vapour or other compounds, which condense from the gas phase, unless they are not directly emitted. Particles are something important in our climate system. They are necessary to form clouds and can also hold back solar radiation from the Earth's surface.
|
Water vapour
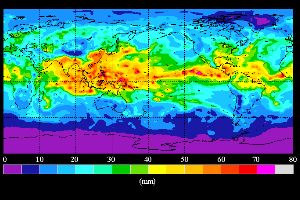
If we summarise the composition of air, we speak about dry air without any water. The main gases (nitrogen, oxygen, and argon) add to nearly 100% of the composition of dry air and indeed there are only a few trace gases left (the most important being carbon dioxide with 0.037%), which make up for the rest. Water vapour, however, is very inhomogeneously distributed in the atmosphere. Depending on the climatic conditions and the temperature the water vapour content of the air may range between 0.1% and 4% in the troposphere and decreases to almost zero anywhere above the tropopause (higher atmosphere, also called stratosphere). Cold air can take up less water vapour than warm air.
|
 |
 |
 |
|
4. Global overview of the total water vapour column in July 1989
Source: NASA water vapour project NVAP
http://www.cira.colostate.edu/climate/NVAP/nvapcira.html
|
|
Trace gases
It is not easy to imagine, but many climate processes depend on trace gases in the atmosphere, which are only present in very low amounts, i.e. a few molecules among one million or even one billion. We use the unit ppm (parts per million) and mean, that 1 ppm is one molecule among 1,000,000. More correct is 1µmol/mol instead of 1 ppm. The fraction of the greenhouse gas carbon dioxide increased from 280 ppm in preindustrial times to about 370 ppm nowadays and increases further on due to human activities, most important of which is fossil fuel combustion. Two other important greenhouse gases are methane (1.7 ppm) and ozone (varying around e.g. 0.04 ppm). In addition there are thousands of organic and inorganic gases which are emitted from plants (imagine the smell of flowers) or during industrial procedures (think about solvents) or are formed during chemical processes in the atmosphere. They contribute to a very complex chemistry, in particular in the lower most layer of the atmosphere, the troposphere.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry - Mainz / Germany
1. sci. reviewing: Dr. Katja Mannschreck - GAW Hohenpeissenberg - 2003-08-07
2. sci. reviewing: Dr. Gerd Folberth - Meteorological Service of Canada / Univ. of Victoria - 2003-08-10
edu. reviewing: Michael Seesing - Uni Duisburg - 2003-07-02
revised and last published: 2004-06-12
|
|
 |
|









