|
|
 |
|
|
|
 |
| |
|
|
 |
Clouds & Particles
More |
Cloud chemistry
Cloud is not an inert mixture of water droplets (or ice crystals) and particles. Particles that allow the cloud’s formation, called CCN (Cloud Condensation Nuclei), have various chemical compositions according to their origin (human or natural: deserts, oceans, volcanoes, or living organisms). The cloud is surrounded with the atmospheric gases, which can also modify the chemical composition of droplets. Thus, a cloud is far from being a quiet thing… |
|
|
|
|
 |
 |
 |
|
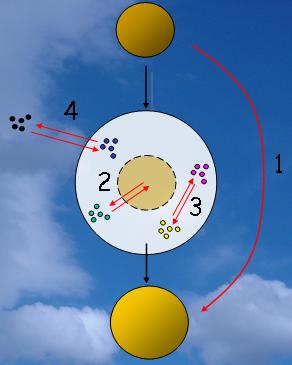
1. The various cloud chemistry processes. Author: J. Gourdeau.
|
|
 |
Four main processes occur within the cloud droplet: the CCN composition and size can change after the droplet has evaporated (process number 1 on the figure 1); dissolution of soluble content of the particle (2) and aqueous reactions inside the water droplet (3); transfers between atmospheric gases and liquid phase (4). |
The particle inside the droplet
The water soluble fraction of an aerosol governs its capacity to grow into a droplet. The chemical composition of the particles that acted as CCN determines the initial composition of the cloud droplet, as its soluble content dissolves in the condensed water. The less hygroscopic particles (that can be for example soil dust, pollen, or particles originated from biomass burning) remain in the surrounding air.
The particle resulting from evaporation of cloud droplets is different from that which entered the cloud, due to in-cloud reactions. |
 |
 |
|
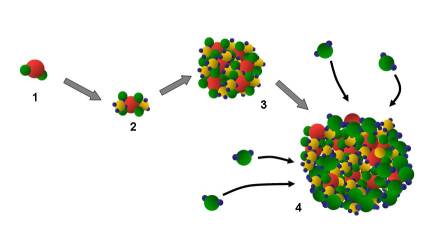
2. A sulfur dioxyde molecule (1) is converted to ammonium sulfate (2) that grows to form an ammonium sulfate particle (3). These particles are hygroscopic, meaning they rapidly grow in the presence of water (4). Author: J. Gourdeau.
|
The atmospheric gases around the droplet
Whether a chemical species stay in the gas phase or is absorbed in the water droplet is estimated by considering the Henry’s law equilibrium: A(aq) = HA PA, where A(aq) is the aqueous phase concentration (mol/L), PA is the partial pressure of A in the gas phase (atm), and HA is the Henry’s law coefficient of the gas considered.
Some species go back to the gas phase and migrate away from the drop; others, once captured, remain associated with the aqueous phase unless total evaporation occurs.
|
Reactions inside the droplet
Not less than about an hundred of chemical reactions take place into the droplet. They are often effective in changing the acidity of the precipitation, leading to acid rains that are hazardous for vegetation and for animals living in lakes and streams, and contribute to the deterioration of buildings. The main chemical species that are involved in acid rain are sulfuric and nitric acids.
|
 |
 |
 |
|
3. A scientist collecting water samples for acid rain analysis. Look at the damaged forest! Source: NOAA.
|
|
|
All this complex chemistry in which a cloud is involved modifies the cloud itself and the atmosphere around the cloud. Just think that only one cloud on seven precipitate and that a CCN is involved in many evaporation-condensation cycles (between 10 and 25!) before it reaches the ground…
|
|
For more details on acid rain click here.
About this page....
Author: J. Gourdeau
Date of generation: 2003-12-4.
1st scientific reviewer: M. Leriche, CNRS LaMP, France. |
|
 |
|









