|
|
 |
|
|
|
 |
| |
|
|
 |
The Oceans
Read more |
The effect of iron and dust on climate
The presence of iron both in the atmosphere and in seawater affects our climate. In the atmosphere, iron containing particles scatter sunlight back into space and cause cooling. The iron in these particles helps to form sulphate aerosols. These sulphate aerosols scatter sunlight back into space and also start the formation of clouds. By influencing phytoplankton growth, iron in seawater affects the ability of the oceans to take up the very important greenhouse gas, carbon dioxide.
|
|
|
|
|
 |
In the atmosphere
Before dust even enters seawater it influences climate. Dust particles in the atmosphere scatter sunlight back into space and cause a cooling at the Earth's surface by preventing the Sun's energy from reaching the ground. Chemical reaction of the iron in the dust with certain sulphur containing species in the air, forms sulphate aerosols (the word aerosol just means a particle or a liquid droplet in air). These aerosols cool the planet both by scattering solar radiation back into space and also by acting as cloud condensation nuclei (CCN). Cloud condensation nuclei are very small aerosols which are needed to start the formation of clouds. Clouds over the oceans also cool the planet, again by scattering sunlight back into space. This is discussed more in Unit 3 in the Basics Section and in the Topic on Clouds and Particles.
|
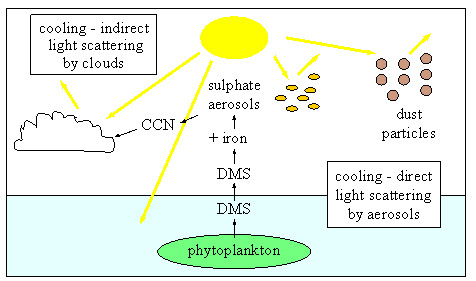 |
 |
|
1. Cartoon of the importance of dust and sulphate aerosols in climate. CCN stands for Cloud Condensation Nuclei, the tiny aerosols which are needed to start the formation of clouds. DMS stands for dimethyl sulphide, a sulphur containing gas which is produced by some species of phytoplankton. This gas is converted to sulphate aerosols in the air and these aerosols affect climate. Author: Lucinda Spokes.
|
Once in seawater
As iron is an essential micronutrient, inputs of iron from the atmosphere are necessary for phytoplankton to grow. We know that phytoplankton take up carbon dioxide (CO2) during photosynthesis and that carbon dioxide is an extremely important greenhouse gas which contributes to global warming. We also know that if the phytoplankton sink out of the surface waters into the deep ocean, they take their carbon with them and, to make up for this carbon removal, carbon dioxide enters the ocean from the atmosphere. This, along with physical carbon dioxide removal processes (see Unit 1 in the Basics Section), reduces atmospheric carbon dioxide levels and, as a consequence, reduces global warming. Some phytoplankton species also produce the gas dimethyl sulphide (DMS) as they grow. This gas is also climatically very important. When it enters the air, it is oxidised to sulphate aerosols which affect climate both by scattering sunlight back into space and also by acting as cloud condenstation nuclei and starting the formation of clouds (see again Unit 3 in the Basics Section and the Topic on Clouds and Particles).
|
 |
 |
|
2. Electron micrographs of diatoms, phytoplankton with silicate shells. Thanks to Ivo Grigorov at SOC, Southampton University, U.K. for use of these images. Click on the image for a better view (27 KB).
|
|
 |
The High Nitrate, Low Chlorophyll (HNLC) regions of the oceans have much less phytoplankton growth than we would predict based on the levels of the major nutrients (nitrate and phosphate) which are present. So low phytoplankton growth in these large areas of the ocean means that, overall, the ocean is a smaller carbon dioxide sink than we initially thought. Lack of iron appears to limit the growth of a particular group of phytoplankton known as the diatoms which are large with a shell made of silicate material. Because of their size, these phytoplankton can sink out of the surface waters in to the deep ocean when they die.
So lack of iron limits the total amount of phytoplankton which grow in the oceans and therefore means that less than expected amounts of carbon dioxide are removed from the atmosphere. It also prevents the growth of diatoms, in particular, and therefore reduces biological removal and storage of carbon in the deep ocean. |
Because a ready supply of iron is critical for phytoplankton growth and phytoplankton are so important to our climate, John Martin suggested that the deliberate addition of iron to the HNLC ocean areas would increase carbon storage in the deep sea and would reduce the impact of global warming. His most famous quote was "give me a tanker load of iron and I will give you the next ice age".
|
 |
 |
 |
|
3. NOAA image of a ship in ice.
|
|
|
In the next section, we look at the pros and cons of deliberately adding iron to the oceans as a way to control our climate.
About this page:
author: Lucinda Spokes - Environmental Sciences, University of East Anglia, Norwich - U.K.
1. sci. reviewer: Dr. Peter Croot - Institute for Marine Research, University of Kiel, Kiel - Germany.
2. sci. reviewer: Dr. Serge Despiau - Faculte des Sciences et Techniques, Universite de Toulon Var, La Garde - France.
edu. reviewer:
last updated: 2003-11-05
|
|
 |
|









