|
|
 |
|
|
|
 |
| |
|
|
 |
The Oceans
Basics |
Phytoplankton and nutrients in the oceans
Phytoplankton (phyto = plant, planktos = to wander) are single celled plants which live in the surface waters of the oceans. Most of them simply drift around the ocean in the surface currents but some can move a tiny bit on their own. They use sunlight, carbon dioxide (CO2) and water, in a process called photosynthesis, to produce organic compounds which they use for food and to make their cells. One waste product is oxygen and this makes it possible for animals to live on earth. Phytoplankton remove almost as much carbon dioxide from the air as land plants and, therefore, help regulate our climate.
|
|
|
|
|
 |
|
|
 |
1. In photosynthesis, chlorophyll, the green pigment in plants, captures energy from the sun. Because phytoplankton need energy from the sun, photosynthesis can only take place in the surface layer of the ocean. In the open ocean, this layer is about 100 meters in depth, above a water column that can be over 3000 meters deep. Some of the sun's energy is used to breakdown water into oxygen and hydrogen. The oxygen isn't needed so this leaves the cell. The hydrogen left when the water breaks down reacts with carbon dioxide and, using more of the sun's energy, forms simple organic molecules such as glucose. These are the building blocks for bigger organic compounds. Author: Lucinda Spokes. |
|
Phytoplankton also need nutrients to grow. They need a wide variety of chemical elements but the two critical ones are nitrogen and phosphorous since they are needed in quite large amounts but are present in low concentrations in seawater. Nitrogen and phosphorous are like the fertilisers we add to land plants and are used to make proteins, nucleic acids and other cell parts the phytoplankton need to survive and reproduce. Phytoplankton need nutrients in well defined ratios. For every 106 atoms of carbon they make into organic matter, they need 16 atoms of nitrogen and 1 atom of phosphorous. Most can't use atmospheric nitrogen gas (N2) directly but need chemically reactive forms of nitrogen such as nitrate (NO3-) or ammonium (NH4+). There is always plenty of carbon dioxide so phytoplankton keep growing until they have used up all of the useable nitrogen or all of the phosphorous, which ever runs out first. In most of the ocean, nitrogen runs out first and growth is said to be nitrogen limited. The Eastern Mediterranean Sea is phosphorous limited, here growth stops when phytoplankton have used up all the phosphorous even though there is still nitrogen in the water.
Nutrient sources
Nutrients come naturally from the weathering of rocks and from the conversion of atmospheric nitrogen gas (N2) into biologically usable forms. Human activity has dramatically added to these inputs.
|
 |  | 2. Main phosphorous sources. Images by Rachel Cave and freefoto.com |
|  | Phosphorous
The main human sources of phosphorous are detergents and sewage. Improved sewage treatment and use of phosphate free detergents has reduced phosphorous inputs to rivers and seas.
Nitrogen
Nitrogen compounds in rivers are mainly the result of intensive agricultural activity and come from the overuse of nitrate (NO3-) based fertilisers and from ploughing up land. Both nitrate and ammonium are found in the atmosphere. Nitrate comes from the high temperature combustion of nitrogen in vehicle engines and during power generation. Ammonium (NH4+) comes from the storage and spreading of animal manure. Both fall from the atmosphere and enter the rivers and oceans in rain and as gases and particles. |  |  |  | 3. Main nitrogen sources. Images by freefoto.com |
|
| | | |  | |
Silicon
Another important nutrient is silicon which comes from the weathering of rocks. Lack of silicon prevents the growth of a certain type of phytoplankton, the diatoms, who use it to make their shells.
If nitrogen or phosphorous runs out, phytoplankton stop growing. If silicon runs out, phytoplankton keep growing but the types which grow changes.
Trace metals
Phytoplankton also need very small amounts of metals such as iron, copper, zinc and cobalt. There are large areas of the oceans where there isn't enough iron for phytoplankton to grow. This has important implications for climate and we discuss this in the Oceanic nutrients Unit of the Read More Section.
|
Remineralisation
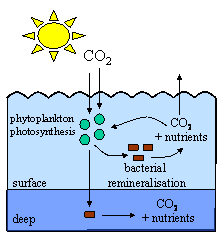
Phytoplankton grow very quickly, only living for a day or so. When they die, they are eaten by bacteria or zooplankton (tiny animals) which convert their organic matter back into carbon dioxide, release the nutrients they have used back into the water and use up oxygen. This process is known as remineralisation and it takes place mainly in surface waters. The carbon dioxide escapes back to the air or is reused, along with the re-released nutrients, in photosynthesis. If this happens, there is no change in atmospheric carbon dioxide levels.
However, if the phytoplankton sink out of the surface and are remineralised in the deep ocean, the nutrients and carbon dioxide are stored in the deep ocean and the carbon dioxide can't return to the atmosphere. This lowers carbon dioxide levels in surface waters, allows more carbon dioxide to enter from the air and helps reduce atmospheric carbon dioxide concentrations.
|
 |
 |
 |
|
4. Very simplified cartoon showing the process of remineralisation. Author: Lucinda Spokes.
|
|
|
The carbon dioxide only returns to the air when ocean circulation brings the deep water back to the surface, a process which takes around 1000 years. This is the biological pump and is explained more in the Water in the oceans Unit in the Basics Section.
About 15% of the carbon taken up by photosynthesis is stored in the deep ocean. A very small part of this settles out and becomes sediments. An even smaller amount eventually becomes oil and coal. By burning fossil fuels, we are releasing this stored carbon about a million times faster than natural biological cycles do. Forests and phytoplankton can't take up the carbon dioxide fast enough to keep up with the increases in emissions and atmospheric carbon dioxide levels have, therefore, risen dramatically over the past few decades.
About this page:
author: Lucinda Spokes - Environmental Sciences, University of East Anglia, Norwich - U.K.
1. sci reviewer: Prof. Tim Jickells - Environmental Sciences, University of East Anglia, Norwich - U.K.
2. sci reviewer: Dr. Keith Weston - Environmental Sciences, University of East Anglia, Norwich - U.K.
edu. reviewer:
last updated: 2003-10-16
|
|
 |
|









