|
|
 |
|
|
|
 |
| |
|
|
 |
Upper Atmosphere
Basics |
Chlorofluorocarbons (CFC's) and the ozone hole
The story of the ozone hole is a good example of how an apparently harmless class of chemical compounds can become a real danger to life on Earth. It also shows how governments, industry and society can work together to identify an environmental problem and solve it.
|
|
|
|
|
 |
|
The ozone hole has taught us that human actions can change the natural state of our climate in unexpected ways. It has also shown us that, if the world community works together in a targetted way, global environmental problems can be effectively dealt with. CFC's are just one class of chemical substance that depletes the ozone layer, but they are the most important one.
|
Properties of CFCs and their use
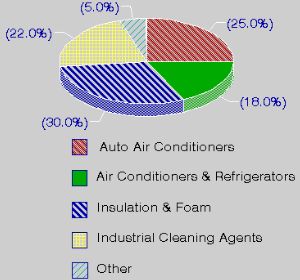
Chlorofluorocarbons are simple organic compounds where all of the hydrogen atoms have been replaced with the halogens chlorine and fluorine (e.g. CFC-11 CFCl3 and CFC-12 CF2Cl2). Commercially they are well known as FREON's. CFC's were used, for example, as refrigerants, as solvents in the electronic industry, as aerosol propellants, in fire extinguishers, as dry cleaning solvents, as degreasing agents and in foam packing and insulation materials.
|
 |
 |
 |
|
1. Use of CFC's. Information from the US Environmental Protection Agency.
|
|
|
CFC's were used extensively because, in the troposphere, they are completely unreactive and do not affect human health in any way. This gives them extremely long atmospheric lifetimes and allows then to accumulate in the atmosphere. The fatal properties of the compounds which humans didn't take into account is that they are broken down by high energy ultra-violet radiation from the Sun.
|
 |
 |
|
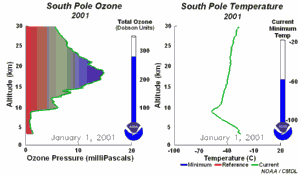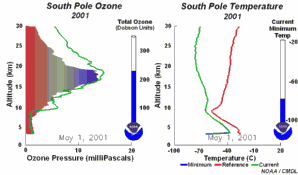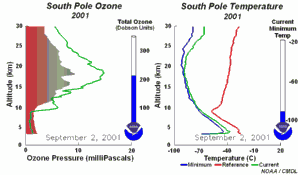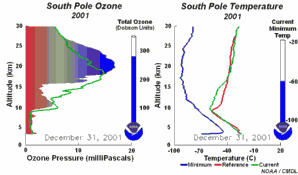
2. The development of the ozone hole 2001. Please click to enlarge a sequence of 5 days (270 K)! Original animation provided by the NOAA Climate Monitoring and Diagnostics Laboratory, Boulder, Colorado.
|
|
 |
The stratospheric fate of CFC's
High energy ultra-violet radiation from the Sun is absorbed by stratospheric ozone and, as a result, doesn't enter the troposphere. This means that the ultra-violet light which does reach the Earth's surface is too weak to breakdown the CFC's present there. However, because CFC's have such a long life time in the atmosphere, significant amounts entered the stratosphere where the ultra-violet radiation was strong enough to break them down into very reactive chlorine and fluorine radical species. These radicals are capable of destroying ozone.
|
|
This process, however, doesn't necessarily lead to strong ozone depletion because chlorine radicals (which are mainly responsible for ozone removal) also undergo other reactions depending on the meteorological conditions. Although stratospheric ozone is removed at all latitudes by reaction with chlorine and fluorine radicals, the ozone hole is only formed at the poles, particularly over Antarctica and only in the spring. Why is this so?
|
How halogen radicals destroy ozone
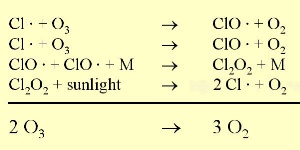
Under natural conditions, ozone levels are relatively constant since they are both formed and destroyed by ultra-violet light. So as ozone concentrations increase, the amount of ozone destroyed also increases. However, chlorine radicals (Cl) react with ozone simply to destroy it. They are very efficient at removing ozone because they act as catalysts. This means that they are not consumed by the reaction but are recycled and can continue to react with other ozone molecules to destroy them as well.
|
 |
 |
 |
|
3. Chlorine ozone reaction. Dots indicate, that the reaction partners are radicals. Full size: 35 K
|
|
The conditions
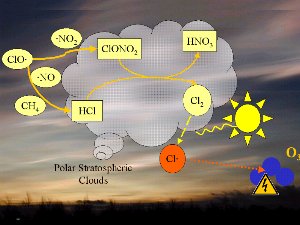
Decomposition of the CFC's leads to chlorine monoxide radicals (ClO). These can then react with nitrogen dioxide (NO2) to form chlorine nitrate (ClONO2) or with nitrogen monoxide (NO) and methane (CH4) to form hydrochloric acid (HCl) and nitric acid (HNO3). We will not focus on the chemistry here, but it is important to know that both HCl and ClONO2 do not react with ozone but are rather stable compounds and remove chlorine from the ozone destruction mechanism.
It is only under certain meteorological conditions that ozone holes form. It took over two years of research at the British Research Station at Halley Bay in Antarctica to finally understand what these conditions are.
|
 |
 |
|
4. Chemistry on polar stratospheric clouds leads to the dangerous chlorine radicals Cl (red).
Please click to enlarge (100 K)!
|
|
 |
1. One factor is the extremely low temperatures in the stratosphere. During the night temperatures can be as low as -80 oC over Antarctica. Under these conditions, nitric acid and water form stratospheric ice clouds. On the surface of the ice, hydrochloric acid and ClONO2 react with each other to form nitric acid and molecular chlorine (Cl2).
2. Molecular chlorine (Cl2) is a stable molecule which does not react with ozone. However, it is easily broken down by ultra-violet radiation from the Sun to form two chlorine radicals which can then attack and destroy ozone. |
So high levels of molecular chlorine (Cl2) can be produced in the stratosphere at the poles during the winter. In the spring, the Sun reappears and levels of solar ultra-violet radiation increase. This ultra-violet radiation breaks down the Cl2 into chlorine radicals, these then destroy ozone and an ozone hole forms. As a result, we see the ozone hole at the same time each year and ozone levels don't recover until the ice clouds thaw and the chlorine radicals are removed by other reactions.
|
 |
 |
 |
|
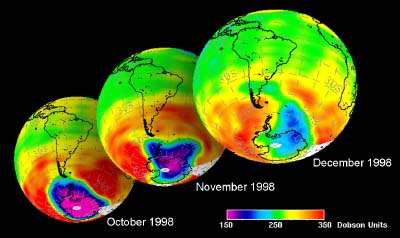
5. Ozone hole development in Antarctic spring 1998. Data from GOME.
|
|
 |
 |
|
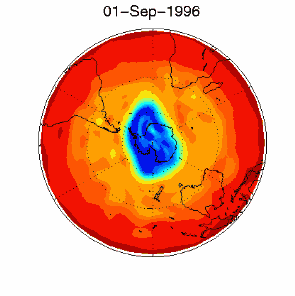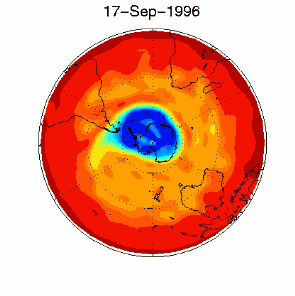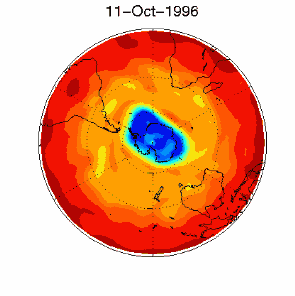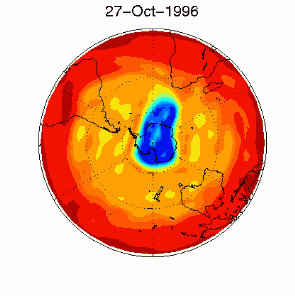
6. The ozone hole in the polar vortex over Antarctica in September / October 1996. Adapted from UKMO data set, published at Brown University. Please click for a higher resolution version (330 K)!
|
|
 |
3. Certain meteorological conditions are then required to allow the ozone hole to develop fully. Formation of the chlorine monoxide radical (ClO) usually occurs at high up in the stratosphere, away from the most of the ozone molecules which are found at altitudes between 14 and 22 km high. So chlorine radicals shouldn't really affect most of the ozone in the stratosphere. This isn't the case, unfortunately. Special meteorological conditions around the poles allows a polar vortex to form and this causes downward air movement. This transports ClO to lower altitudes where it plays a part in ozone destruction. |
|
As you can see, the conditions required to form the ozone hole:
- cold temperatures during the polar winter
- ice cloud formation
- special meteorological conditions to form the polar vortex
- followed by the polar sun rise in the spring
are so special that it is really unlikely that scientists could ever have predicted the ozone hole.
|
The future of the ozone hole
CFCs were banned globally by the Montreal Protocol on substances that deplete the Ozone layer-1987 (and further amendments). As they have such long atmospheric lifetimes (the longest lived have lifetimes of the order of 100 years) it will take around another 50 years until all the CFC's released so far have been destroyed in the stratosphere and ozone concentrations stabilise. Maximum CFC concentrations in the stratosphere were predicted to occur in 2000 and the ozone hole has been rather constant in size over the past few years. However, there are exceptions to this trend. In 2002 no significant ozone hole was seen. The reason why was simple: it was too warm and the polar vortex did not form as usual. Once again, an example that atmospheric processes sometimes ignore any prediction! But in 2003 the hole was back to its previous size, the second largest ever observed.
|
 |
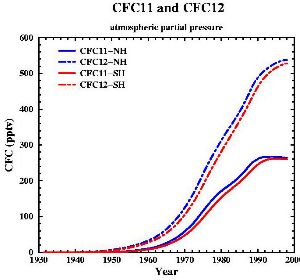 |
 |
|
7. Developement of the concentrations of the two most important CFCs (also called FREON 11 and FREON 12). Data from: Walker et al., J. Geophys. Res., 105, 14,285-14,296, 2000 [internet plots]. Picture by Gian-Kasper Plattner (Univ. of Bern, UCLA).
Please click to enlarge!
|
|
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany.
1. scientific reviewing: Rajendra Shende, Head Energy and OzonAction, United Nations Environment Programme 2003-10-06
2. scientific reviewing: Dr. John Cowley - Max Planck Institute for Chemistry, Mainz - 2004-05-06
educational proofreading: Hendrik Forster and students - Nordpfalz Gymnasium, Kirchheim Bolanden, Germany - March 2004
last published: 2004-06-22
|
|
 |
|







