|
|
 |
|
Ozone is very important in our stratosphere because it absorbs harmful ultra-violet radiation coming from the Sun and prevents it reaching the surface of the Earth. This experiment (E) and tasks (T) will help you understand the important role of ozone in the stratosphere.
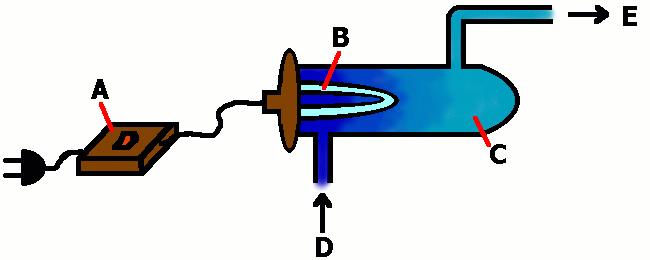
The equipment shown in Figure 1. is used to make ozone. The reactions which occur in the chamber are the same as those in the Chapman Cycle in the stratosphere. A special medium pressure mercury vapour lamp is needed for the experiment.
|
 |
 |
|
fig. 1 formation of ozone 1. © 2003 Seesing, Tausch; Duisburg
A: fluorescent lamp ballast
B: ____________lamp
C: reactor
D: oxygen
E: ozone
|
Explain briefly why a normal incandescent lamp can't be used to form ozone.
Enter the necessary characteristics of lamp in part "B" of the legend to Figure1.
CARE: The chemicals, oxygen and ozone and the short wave ultra-violet radiation are harmful. Experiment carefully and protect yourselves by using safety equipment. Wear special safety glasses and cover the UV-light-reaction chamber with aluminium foil to protect your eyes.
You need a room which can be made dark and the following materials:
|
|
1
1
1
|
equipment to produce ozone
fluorescent screen, or a TLC-foil with fluorescent indicator F254
UV lamp (wavelength =254 nm)
glass tubes to pass the ozone through
aluminium-foil (to darken and as protection from ultra-violet radiation) |
|
 |
 |
 |
|
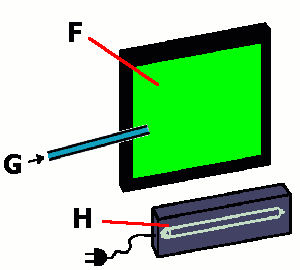
F: fluorescent screen (wavelength=254nm)
G: ozone (oxygen mixture)
H: UV lamp (wavelength=254nm)
2. © 2003 Seesing, Tausch; Duisburg
|
|
Form ozone and drive it in short pulses through a glass tube between the fluorescent screen and the UV lamp.
Alternatively, click on the picture and watch a short film of the experiment [OzoneAndLight.avi 192kB].
Describe briefly what you see .
What is the reason for this?
Why is this behaviour very important for life on Earth? |
|
|
 |
Download |
|
 |
 |
 |
3. Movie: OzoneAndLight.avi (192kB) |
|
About this page:
authors: M. Seesing, M. Tausch - Universitšt Duisburg-Essen, Duisburg, Germany
last update: 2004-05-24
|
|
 |
|







