|
|
 |
|
|
|
 |
| |
|
|
 |
Upper Atmosphere
Basics |
Formation of ozone
If it wasn't for stratospheric ozone, life as we know it now wouldn't be possible on Earth. Ozone prevents harmful ultra-violet radiation from the Sun (light with wavelengths less than 320 nm) reaching the ground. If allowed to reach Earth, this radiation would severly damage the cells that plants and animals are made up of. Ozone was first formed in the Earth's atmosphere after the release of oxygen, between 2000 and 600 million years before the first humans appeared.
|
|
|
|
|
 |
 |
 |
|
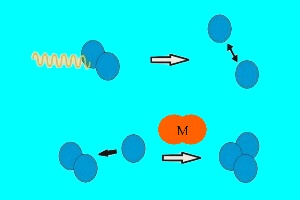
1. Ozone formation - ultra-violet radiation from the sun (shown in yellow) splits oxygen molecules into oxygen atoms which then react with more O2 to form ozone. Another molecule (M) is needed to absorb some of the huge amount of energy involved in the reaction.
|
|
 |
Ozone formation and destruction
Two forms of oxygen are found in the stratosphere. Molecular oxygen (O2), which is made up of two atoms of oxygen (O), and ozone (O3) which, as you can see from its chemical formula, is made up of three oxygen atoms. Ozone is formed when intensive ultra-violet radiation from the Sun breaks down O2 into two oxygen atoms. These highly reactive oxygen atoms can then react with more O2 to form O3.
|
 |
 |
|
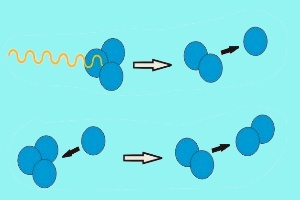
2. Destruction of ozone by solar radiation.
|
|
 |
In a similar way, ozone is destroyed by solar radiation. Ultraviolet radiation hits ozone and breaks it back down into molecular oxygen (O2) and atomic oxygen (O). The oxygen atom O then reacts with another ozone molecule to form two oxygen molecules.
|
Formation in the tropics, accumulation in polar regions
Since solar radiation is strongest over the tropical regions, most of the ozone is formed in there. The Sun doesn't just drive this tropical ozone formation but also allows tropospheric air to rise to higher altitudes here.
|
Even though ozone is produced in the tropics, concentrations aren't particularly high since intensive solar radiation means that ozone loss in this area is also high. Ozone is transported from the tropics towards the poles where it accumulates. Ozone can accumulate in these regions as solar radiation isn't strong enough here to cause much ozone destruction. Unless disturbed by the formation of the Antarctic ozone hole in spring, ozone levels are generally highest in the cold subpolar regions. Concentrations are slightly lower at the poles themselves, particularly in winter, as no additional ozone can be formed here because there isn't any sunlight!
|
 |
 |
 |
|
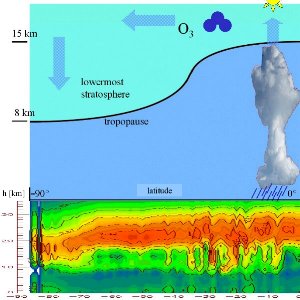
3. This scheme shows ozone transport. The simulation below shows how measured ozone concentrations vary between the poles and the equator (low values = blue, high values = red). It also shows air containing low ozone levels rising at the Equator to high latitudes. Data from GOME (DLR, IUP Bremen).
Please click to enlarge (100 K)!
|
|
Ozone absorbs ultra-violet radiation
Absorption of the Sun's ultra-violet radiation during ozone formation and destruction in the stratosphere has three important consequences.
|
 |
 |
|
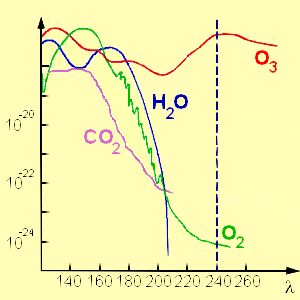
4. Absorption of ultra-violet radiation by ozone and other compounds in the stratosphere.
Adapted from Chemie Didaktik Uni Duisburg.
|
|
 |
- less ultra-violet radiation reaches the lower parts of the atmosphere and, as a consequence, the surface of the Earth is protected from damaging radiation.
- since ultra-violet radiation is important for both ozone formation and destruction, the amount of ozone that can accumulate is limited - as ozone formation increases, ozone destruction also increases.
- ultra-violet radiation is highly energetic. This energy is transformed to heat during reaction leading to a warming of the stratosphere. This is the reason why the temperature trend in the stratosphere is opposite to that seen in the troposphere.
|
Thickness of the ozone layer
The term "ozone layer" is often misunderstood. Its not really a single layer in the atmosphere where ozone is concentrated. Rather it means that a higher fraction of ozone molecules are found in the stratosphere (at altitudes between 18 and 40 km) compared to levels the troposphere below or the mesosphere above. In reality, only about 10 out of every million molecules in the atmosphere are actually ozone but 90% of all the ozone present in the atmosphere is found in the stratosphere.
|
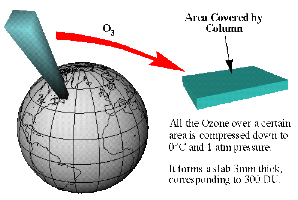
Dobson units (DU)
You will often see ozone levels reported in Dobson units (DU). 300 DU is a typical value. But what does this mean? If we assume that all the ozone molecules in the atmosphere were concentrated in a small layer at the ground (rather than being spread over the whole stratosphere and troposphere) then then thickness of this layer would be about 3 mm. Since 1 DU is equivalent to a layer of pure ozone molecules 0.01 mm thick, a 3 mm layer of ozone is equivalent to a value of 300 DU.
|
 |
 |
 |
|
5. Visualisation of a Dobson Units
source: Univ. of Cambridge Ozone Hole Tour.
Please click to enlarge! (20 K)
|
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany.
scientific reviewer: Dr. John Cowley - Max Planck Institute for Chemistry, Mainz - 2004-05-06
educational proofreading: Henrick Forster and students - Nordpfalz Gymnasium, Kirchheim-Bolanden - March 2004
last published: 2004-05-10
|
|
 |
|







