 |
 |
|
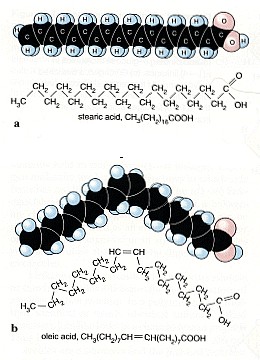
2. Not all fatty acids have double bonds. Some are (a) saturated and so just have C-C single bonds. Many of them are, however, (b) unsaturated and have C=C double bonds. Source: NTRC, Univ. of Texas Kingsville.
|
|
 |
Ozone attacks our respiratory system
We know that organic compounds made up of carbon (C) and hydrogen (H) are present in all living organisms, in plants, in animals and in our bodies. As well as C and H, these compounds may also contain oxygen (O), nitrogen (N), sulphur (S) and phosphorous (P). The main structures of organic compounds are made of carbon, and generally include C-C single bonds and C=C double bonds. C=C double bonds are found everywhere; in unsaturated fatty acids, haemoglobin, proteins, on the surface of the pulmonary alveoli in our lungs and its mucus membrane and in many other bio-molecules. Ozone attacks these C=C double bonds.
The other two main oxidants, OH and NO3, have extremely short lifetimes and react immediately they are formed. Ozone, however, can enter the lungs. Every day we breathe 20,000 liters of air into our lungs. This air carries many chemicals and particles, including ozone.
|







