|
|
 |
|
|
|
 |
 |
The Atmosphere
The air surrounding us
|
Although you cannot see anything, air is made up of matter. As skydivers show, you can dive in air in the same way as you can dive in water. The resistance is much smaller but it is still there. The air surrounding us consists of countless invisible molecules and atoms floating in empty space. These molecules and atoms interact with radiation from the sun and with each other, starting off chemical reactions. Bigger molecules can also stick to each other and form little particles. Water molecules can condense onto these particles and form cloud droplets. If more and more cloud droplets are formed and grow they finally form clouds which appear in the sky.
|
 |
 |
 |
|
1. Skydivers - air is more than nothing
© Skydivers Sittertal
|
|
 |
 |
|
2. Imagine air molecules as little spheres floating quickly through the air.
Click on the picture in order to see a Java simulation of the motion of air molecules! A new page will open you can close afterwards.
|
|
 |
Composition
If we don't include water, the air around us is made up of about:
78.08 % nitrogen (N2)
20.95% oxygen (O2)
0.93% argon
These three gases make up 99.96% of dry air. What about carbon dioxide, methane, ozone, hydrocarbons and other gases in the air you have probably heard about? Carbon dioxide is the most abundant of these trace gases and makes up about 0.037% of the air. All other gases are found in smaller amounts. For every million air molecules, you will usually find less than one molecule of these other gases. Nevertheless, they are extremely important for our climate.
|
Dimension of the atmosphere
Looking into the sky we can easily overestimate the thickness of the atmosphere, it is actually a very thin layer above the Earth. If we travel in a plane at an altitude of between 11 and 12 km (that's between the troposphere and the stratosphere), about 75% of the molecules in the air are below us. This means that an atmospheric layer, less than one thousandth of the Earth's diameter (12800 km), contains three quarters of the mass of the whole atmosphere. In comparison, this is equivalent to a layer of snow less than one centimetre thick on a four storey building. In this layer, clouds form and all our weather takes place.
|
 |
 |
 |
|
3. The atmosphere, appearing in light blue at the horizon, is a very thin layer
source: adopted from STRATO foto archive
|
|
 |
 |
|
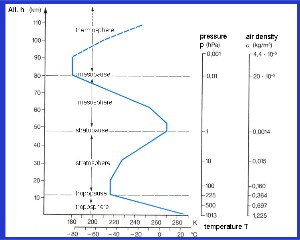
4. The temperature profile, presssure and densitity of the air with increasing altitude
adapted from: Schirmer - Wetter und Klima - Please click to enlarge! (120 K)
|
|
 |
The temperature profile and atmospheric layers
We cannot see the layers in the atmosphere with our eyes but we can measure several changes in the temperature trend as we go from the Earth's surface to higher altitudes. These changes in temperature trends define the atmospheric layers:
The troposphere, the lowest layer of the atmosphere, where temperature decreases with height.
In the stratosphere, the upper atmosphere, temperature increases with height.
Above the stratosphere is the mesosphere, here temperature decreases with height.
The thermosphere is the highest layer in the atmosphere and temperature increases with height here.
|
|
Between the layers there are certain points where the temperature trends change. They are known as '-pauses'. So between the troposphere and the stratosphere we have the tropopause. Above the stratosphere there is the stratopause. The mesopause is found between the mesosphere and the thermosphere and this is the coldest point in the atmosphere. Temperatures here can be as low as
-100 oC!
In contrast to the temperature, the pressure and density of the air decreases continuously with altitude as the air gets thinner. For a fixed volume of air, there will be 1000 oxygen molecules near the surface of the Earth but at an altitude of 50 km, the same volume of air will contain just one oxygen molecule.
|
The interaction of light and air
The energy of the sun is taken up by the surface of the Earth, by the land and even more by the oceans. They convert it into heat and, as a result, the further away we are from sea level, the lower the temperature. This process is what controls the temperature trend in the troposphere, the lowest layer of our atmosphere.
What causes this temperature trend to change direction in the stratosphere? The air becomes warmer if air molecules can absorb energy from the sun themselves. In the stratosphere it is the ozone molecules of the ozone layer which take up the energy. The properties of such molecules are extremely important to our climate. The amount of energy a molecule absorbs depends on the molecule itself and on the wavelength (energy) of the light.
|
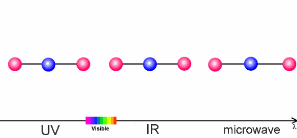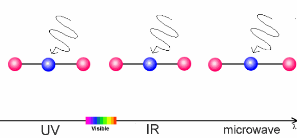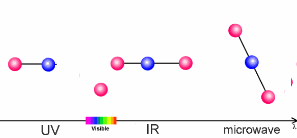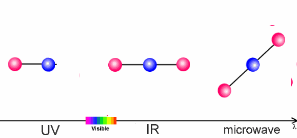
The animation on the right shows three examples:
- Microwaves have only a little energy. They make molecules rotate, but can't break chemical bonds.
- Infrared radiation (IR) is a little bit stronger. It makes molecules vibrate. The atoms swing arond and bond lengths change. This happens when greenhouse gases take up the heat radiation emitted from the Earth's surface.
- Ultraviolet light (UV) has even more energy and can break chemical bonds. This is what happens if ozone in the ozone layer absorbs energy from the Sun.
|
 |
 |
 |
|
5. Sun light interacts with molecules in the air. Depending on the energy of the light, it may break bonds (ultraviolet light), make the molecules swing (infrared light) or if the energy is low, only rotation is initiated.
Click on the image to see it in bigger size (75 K)
© Ecole normale superieure de Lyon
http://www.ens-lyon.fr/Planet-Terre/Infosciences/Climats/Rayonnement/Cours/
|
|
|
The absorption of energy from the Sun by ozone in the stratosphere keeps the Sun's energy in this layer and is the reason why the temperature of the stratosphere increases with altitude. This is similar to the process which occurs in the thermosphere but here it is oxygen and nitrogen which absorb even more energetic radiation from the Sun. The radiation is so energetic that it does not only break bonds but forms charged atoms and molecules known as ions. This is why the layer is also known as the ionosphere.
|
|
About this page:
author: Dr. Elmar Uherek - MPI for chemistry, Mainz
last published: 2004-07-13
|
|
 |
|









