|
|
 |
|
|
|
 |
 |
The upper atmosphere
Characteristics of the stratosphere
The atmospheric layer above the troposphere is known as the stratosphere. It extends from about 15 kilometers to about 50 kilometers in altitude.
The temperature in the stratosphere increases with height. But this isn't because the Sun is closer to the stratosphere compared to the troposphere! It is because the stratosphere contains high concentrations of the gas ozone (O3) which absorbs ultraviolet radiation from the Sun and converts it to heat. This prevents the ultraviolet radiation getting to the Earth's surface. As a result, this ozone layer is very important to our climate.
|
 |
 |
 |
|
Enyclopaedia
Link to topic
Upper Atmosphere
|
|
Very little air moves between the troposphere and the stratosphere so the stratosphere contains hardly any water. This means that stratospheric clouds only form if it is so cold that the tiny amount of water present can condense to form ice crystals.
|
 |
 |
 |
|
1. Stratospheric clouds above Kiruna / Sweden
Source: MPI für Kernphysik / Heidelberg
|
|
|
|
 |
Only chemicals with very long atmospheric lifetimes can reach the stratosphere. However, once in the stratosphere they can remain there for a long time. Material emitted from large volcanic eruptions, such as El Chichon in 1982 and Mount Pinatubo in 1991, stayed in the stratosphere for up to two years.
Aviation
Aeroplanes generally fly between 10 and 12 kilometres in altitude. Increased aviation traffic has, therefore, led to higher emissions of carbon dioxide (CO2), water vapour (H2O), nitrogen oxides (NOx), sulphur oxides (SOx) and soot carbon into the atmosphere between the upper troposphere and the lower stratosphere.
|
At the moment planes account for about 2-3% of global greenhouse gas emissions and this is predicted to rise to 3-4% of total emissions in the future. What is really important is that they emit the gases high up in the atmosphere. The water vapour released increases the likelihood that high cirrus clouds will form. These trap heat from the Earth and so encourage global warming. Nitrogen oxides released from aeroplane exhausts are also important to our climate, these are involved in stratospheric ozone depletion.
|
 |
 |
 |
|
3. Aviation - an increasing burden for the atmosphere
Airbus A320 - by Ian Britten © FreeFoto.com
|
|
 |
 |
|
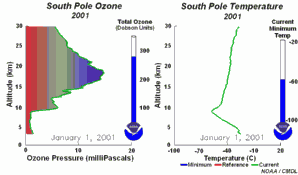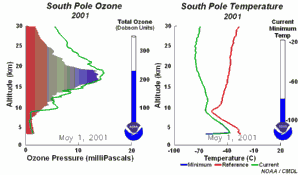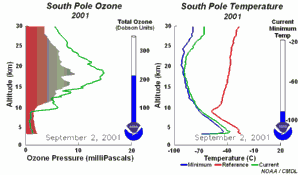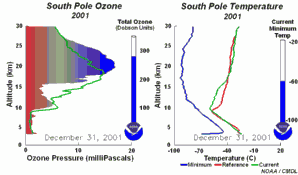
4. The development of the ozone hole 2001. The left part of the animation shows in which altitude ozone over Antarctica is depleted and recovers again during the year. The right part shows the temperature profiles in parallel.
Please click to enlarge and to see a sequence of 5 days (270 K)!
Original animation provided by the NOAA Climate Monitoring and Diagnostics Laboratory, Boulder, Colorado
|
|
 |
The importance of the ozone layer.
Ultraviolet radiation (with wavelengths between 220-320 nm) reaches the Earth and can cause skin cancer if doses are high enough. Fortunately there are high concentrations of ozone in the stratosphere at altitudes between 20 and 45 kilometres. Much of the ultraviolet radiation from the Sun is absorbed by this ozone layer and converted to heat. This protects us from the damaging rays of the Sun.
|
The ozone hole
In the stratosphere, formation and degradation of ozone takes place at the same time. Ozone is formed when sunlight breaks an oxygen molecule (O2) into two oxygen atoms (O). One of the free oxygen atoms then reacts with another oxygen molecule to form ozone (O3).
Ozone is naturally broken down by light back into oxygen atoms (O) and oxygen molecules (O2). The oxygen atom then reacts with another molecule of ozone to form two oxygen molecules. This natural cycle of ozone formation and depletion used to be in balance and ozone concentrations in the stratosphere stayed relatively constant.
|
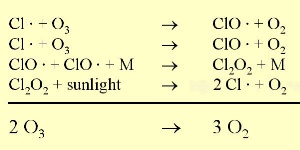
However, there is an another way by which ozone is lost in the stratosphere. Chloroflurocarbons (CFC's) are very stable in the troposphere and can, therefore, reach the stratosphere. Once in the stratosphere they are broken down by ultraviolet light into highly reactive chlorine radicals. These destroy ozone. The same occurs with bromine radicals even more efficiently. Unfortunately, due to human acitivity, high amounts of chlorine and bromine containing compounds such as CFC's have been transported to the stratosphere.
|
 |
 |
 |
|
5. Ozone depletion in the stratosphere
|
|
 |
 |
|
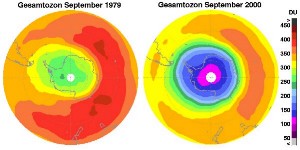
6. The ozone hole in 1979 compared to 2000. Ozone values are given in Dobson Units (DU) decreasing from red to violet.
Source: IUP Bremen.
|
|
 |
This extra ozone destruction mechanism means that ozone levels have fallen in the stratosphere. Because these extra reactions need light and the formation of polar clouds at very low temperatures, ozone levels are lowest in the Antarctic spring and an ozone hole forms in particular over the Antarctic continent. However, low ozone levels have also been seen over the Arctic. These ozone holes mean that more ultraviolet radiation from the Sun now reaches the surface of the Earth. Human health suffers as a result of the increased incidence of skin cancer and plants are also damaged.
|
|
Have a look at the section on the UPPER ATMOSPHERE in the Climate Encyclopaedia to find out more about the layers in our atmosphere and the ozone layer in particular.
About this page:
Author: Dr. Elmar Uherek - MPI for chemistry, Mainz
English proof reading: Lucinda Spokes, UEA Norwich - Sally Taylor, Univ. of Leeds
last published: 2005-06-14 |
|
 |
|







