|
|
 |
|
|
|
 |
 |
The lower atmosphere
The lowest layer of the atmosphere, which is known as the troposphere, extends from the Earth's surface up to the top of the highest clouds you usually see. In this region of the atmosphere, the air becomes colder as the altitude increases. Between the troposphere and the next layer of the atmosphere, the stratosphere, is the tropopause. The tropopause is simply a temperature minimum between the two layers and its altitude varies around the Earth. The tropopause is typically about eight kilometres above the Earth in polar regions and about 15-18 kilometres high in the tropics.
|
 |
 |
 |
|
Encyclopaedia:
Link to topic
Lower Atmosphere
|
|
Water vapour
If we define the composition of air, we mean air which contains no water. If we exclude water, air composition is nearly the same all over the world. The amount of water in the air varies greatly and depends strongly on the air temperature. Really cold air is about 0.1% water whereas very warm air can hold much more water, up to 4% of its mass. At the North and South Poles and in the upper troposphere where it is very cold, the air contains less than one gram of water per kilogram of air. In the tropics where it is warm, air contains up to 30 grams of water per kilogram of air. This huge variability makes it extremely difficult to include water in global climate models.
|
 |
 |
|
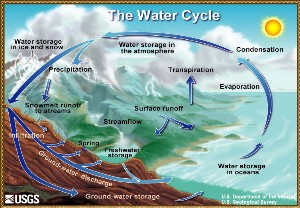
3. The water cycle
source: US geological survey, Illustration by John M. Evans USGS, Colorado District
Please click the image to see a larger view! (150 K)
|
|
 |
We have to keep in mind a few rules in order to understand basic processes in the lower atmosphere:
1) Warm air rises. This is why air mixes in the morning when the Sun warms the ground.
2) Water evaporates and rises to higher (= cooler) regions with the rising air.
3) Cooler air cannot take up so much water. It condenses, forms clouds and may start raining.
4) A temperature minimum occurs at the tropopause. Water and air stop rising here. Only a very small fraction of the water and other chemicals released in the troposphere can get across the tropopause into the stratosphere. |
|
As a consequence: Weather (evaporation, cloud formation, rain, snow) and most of the chemical processing of compounds from the oceans, from land and from human activities takes place in the troposphere.
|
The Greenhouse Effect and global warming
Life on Earth would not be possible without a natural greenhouse effect. Without greenhouse gases, the Earth would be about 33°C cooler and the average temperature would be -18°C instead of 15°C. Water vapour and carbon dioxide are the most important greenhouse gases. Water vapour makes up about 60% of the natural greenhouse effect, and carbon dioxide about 20%. Greenhouse gases trap heat emitted from the Earth and keep it close to the surface. The following image shows what controls the 'central heating' system of our planet.
|
 |
 |
|
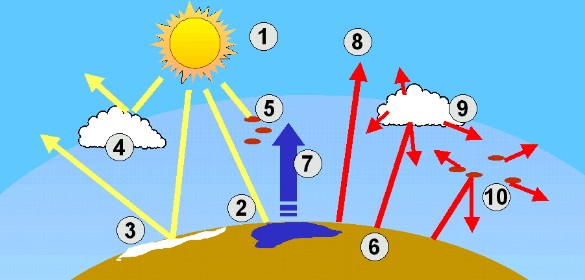
4. The world's energy and radiation system
author: Elmar Uherek
|
|
(1) The Sun is the source of all of the energy reaching the Earth.
(2) Sunlight falls on all of the Earth's surface.
(3) The Earth's surface does not take up all of the Sun's energy. Part of it is reflected directly back into space. Very light coloured surfaces (e.g. ice and snow) are excellent reflectors.
(4) Some sunlight is reflected back into space by the top of clouds.
(5) Gases and particles in the air take up (absorb) sunlight.
(6) The surface of the Earth absorbs radiation from the Sun. This radiation is re-emitted as heat (long wave infrared radiation), warming the Earth.
(7) A bit of the energy absorbed is required to make water evaporate.
(8) A very small amount of the infrared radiation goes directly back into space.
(9) Clouds not only reflect sunlight, they also absorb the heat radiation from the Earth. A cloudy sky keeps the Earth warm, like a blanket.
(10) There are particles and gases in the air which absorb the infrared radiation emitted from the surface of the Earth. The gases are called greenhouse gases, because they trap the heat energy near the ground.
Which gases act as greenhouse gases and what causes the enhanced greenhouse effect?
|
 |
 |
|
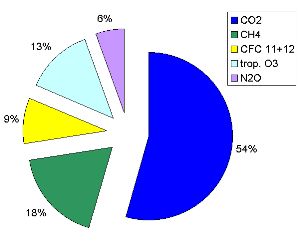
5. The contribution of different greenhouse gases to the additional man made greenhouse effect.
source: Data from IPCC TAR, diagram by Elmar Uherek
Please click to enlarge! (50 K)
|
|
 |
The most important greenhouse gases influenced by humans:
- carbon dioxide (CO2) comes mainly from fossil fuel burning.
- methane (CH4) is produced by cows, sheep and other ruminants and emitted from rice fields, landfill sites and oil deposits.
- chlorofluorocarbons (CFC's) are used in cooling systems and as propellants, foams and cleaning agents.
- tropospheric ozone (O3) comes mainly from industry and traffic.
- nitrous oxide (N2O) is produced during microbial activity in soils and emissions rise when the land is fertilised.
|
Chemistry
Many chemical reactions occur in the troposphere. The chemicals come from many sources including plants, industry, vehicles and the oceans. Nearly all the organic (carbon based) compounds in the troposphere react with one or more of the following species. These are the main atmospheric oxidants and they clean the air of harmful chemicals.
- the hydroxyl radical - OH
- the nitrate radical - NO3
- and ozone - O3.
|
Hydroxyl radicals are formed by the action of sunlight and are extremely reactive so don't exist for very long in the atmosphere. Since they react with nearly all other chemicals, they are known as the detergent of the atmosphere. Nitrate radicals are formed in the dark and broken down by sunlight. These, therefore, clean the atmosphere during the night. In order to form these compounds and ozone, three 'ingredients' are necessary: oxygen, sunlight and the nitrogen oxides, nitrous oxide (NO) and nitrogen dioxide (NO2). These two compounds are collectively known as NOx.
|
 |
 |
 |
|
6. OH - detergent of the atmosphere
|
|
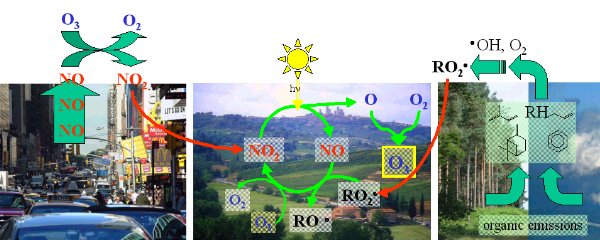
Ozone smog
High concentrations of ozone can be formed in the troposphere during ozone smog events. Since ozone is harmful to human health and to plants, many studies have been conducted to see how it forms.
|
 |
 |
|
7. The formation of ozone smog
author: Elmar Uherek - Click the image for a full size view! (100 K)
|
|
Have a look in the topic on the LOWER ATMOSPHERE in the Climate Encyclopaedia for more details on the Greenhouse Effect, on tropospheric ozone and other subjects including vegetation fires.
About this page:
Author: Dr. Elmar Uherek - MPI for chemistry, Mainz
English proof reading: Lucinda Spokes, UEA Norwich - Sally Taylor, Univ. of Leeds
last published: 2005-06-14 |
|
 |
|







