|
|
 |
|
|
|
 |
 |
|
|
 |
How forest smell forms particles and allows clouds to grow |
Hyytiälä
Hyytiälä is a research station in the Finnish forest. Here, atmospheric scientists from the  Aerosol research group of Helsinki University, in co-operation with many other scientists, try to understand how particles (also called aerosol) form and what role they play in cloud formation. Aerosol research group of Helsinki University, in co-operation with many other scientists, try to understand how particles (also called aerosol) form and what role they play in cloud formation.
|
 |
 |
 |
|
1. View from Hyytiälät research tower above the forest
Source: ISAS Dortmund SMEAR II campaign
|
|
 |
 |
|
2. morning light
|
|
 |
In the morning, when the sun rises, trees in the forest begin to be biologically active. They release many chemical compounds like isoprene or monoterpenes as gases to the atmosphere. Such organic chemicals give the typical flavour to the air in the forest.
Oxidation and particle formation
The organic compounds are oxidised in the air, for example by OH radicals. Often the oxidised compounds have a much lower vapour pressure than the gases originally released by the plants. They tend to condense.
|
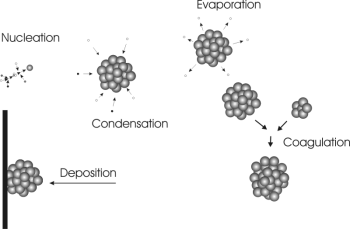
Such low volatile compounds can either deposit on surfaces or stick to each other in the air, form clusters of several molecules and grow to particles. We call this process nucleation. Several clusters can stick to each other and form larger particles. This process if called coagulation. Molecules can evaporate again from such particles, others can condense on them.
|
 |
 |
 |
|
3. Particle formation © Ari Asmi
Please click the picture for a larger version! (50 KB)
|
|
 |
 |
|
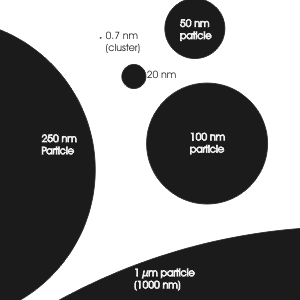
4. Comparison of typical particle sizes from the first small clusters to larger particles of 1 µm. A human hair is 20-100 µm thick. © Ari Asmi
Please click to enlarge!
|
|
 |
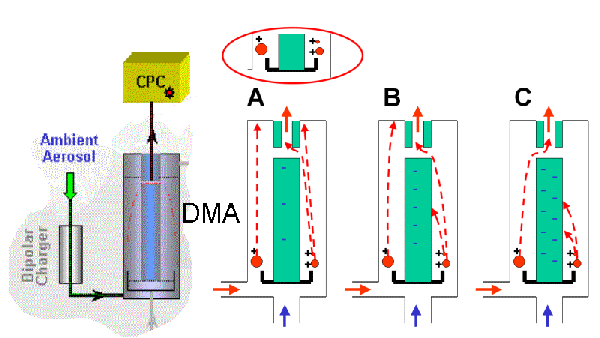
If we compare particle sizes, it is hard to imagine that such little particles can be detected at all. To separate particles by size, scientists use instruments that deflect charged particles in an electric field. The smaller the particles, the more mobile they are and the easier to deflect. Coming out of such a so called 'differential mobility analyser' (DMA), vapour is condensed on them so that they grow up to a detectable size. In a nutshell, detection of very small particles takes place in three steps: 1) charging 2) separation 3) growth + detection.
|
 |
 |
|
5. Particle analysis: Particles are charged, separated and counted (CPC - condensation particle counter). The schemes on the right show how particles (red) of three different sizes are selected by changing the strength of the attracting field (increasing from A to C).
Scheme: Elmar Uherek, Please click to enlarge (50 KB)
|
|
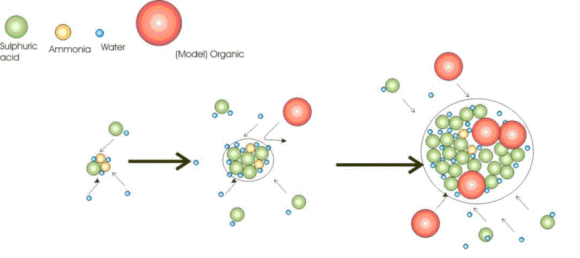
Compared to large organic molecules or salts, pure water is a very volatile species. Water molecules cannot really form clusters in the air, stick to each other and form cloud droplets. A water cluster, if formed, would evaporate again. Water condenses on the tiny particles (aerosols), which are already floating in the air. If more and more water molecules condense on them, a droplet is formed. The condensation process depends very much on if the particles like water or not, i.e. on their chemical composition.
|
 |
 |
|
6. Growing process of particles, scheme: Ari Asmi
Particles consist of single molecules with certain chemical properties. The chemical composition of the smallest particles can vary:
Sulphuric acid and ammonium sulphate particles are found nearly everywhere in the air and easily attract water. They are also found in very clean air over the ocean. Therefore, we think clouds over the ocean can form mainly from water condensing on sulphuric acid. Over land we have higher amounts of large organic molecules coming, for example, from the molecules of the forest smell. Here, as shown in a model above, such organic particles may either condense on sulphuric acid and ammonium sulphate, or perhaps they also condense on themselves.
|
Today, particles of less than 1 nm can be detected and nucleation events observed. Here the biological activity of the forest plays a role. On the graphs above we see how high concentrations of very small particles are measured in the morning hours in the forest of Hyytiälä by a particle analyser. Most of the material comes from the trees and it may have an influence on the kinds of clouds formed on this spring day in 2003.
9. Measurement tower for analysis of forest air in different altitudes in Hyytiälä.
source: ISAS Dortmund SMEAR II campaign
Click on the photo for a larger view (65 KB)
Author: Elmar Uherek
Max Planck Institute for Chemistry Mainz
Acknowledgement:
Many thanks for support in this article to Asbjörn Aarflot, Boris Bonn, Ari Asmi and other colleagues in the research group of Prof. Markku Kulmala in Helsinki.
last update: 2005-05-05
|
 |
|
|
 |
|







