|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more
! significantly changed !
2004-05-07 |
Oxidation in the Atmosphere
Numerous chemical compounds are emitted to the atmosphere and would accumulate if they would not be removed again. Removal can occur via dry deposition or wash out by rain (wet deposition). In particular for gaseous organic compounds removal from the atmosphere is easier if they are transferred into a less volatile, water soluble oxidised state ...
|
|
|
|
|
 |
 |
 |
|
1. OH cleans the air
image: Elmar Uherek
|
|
 |
Oxidation in a chemical sense does not necessarily mean a reaction with oxygen containing compounds. But in the air in most cases oxygen is involved. There are three major oxidants which govern such processes in the atmosphere:
the hydroxyl radical OH
the nitrate radical NO3
the ozone molecule O3
Also HO2 radicals are important and sometimes the sum of OH and HO2 is called HOx. The most important oxidant however is the hydroxyl radical OH. It is extremely reactive and able to oxidise most of the chemicals formed in the troposphere.
Therefore OH is called the 'detergent of the atmosphere'.
|
Only a few compounds like chlorofluorocarbons CFCs (for example CF2Cl2), nitrous oxide N2O or carbon dioxide CO2 are very stable and do not react at all or react very slowly with OH. Also the reaction rate of methane CH4 is 100-1000 times lower compared to other organic compounds. This explains why methane concentrations (about 1.7 ppm = 1.7 µmol/mol) can be rather high in the atmosphere, while most of the organic trace gas concentrations range below 1 ppb (= 1nmol/mol)*.
|
 |
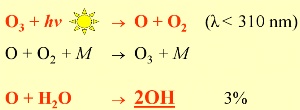 |
 |
|
2. Formation of OH: >97% of the O atoms, formed by photolysis of ozone, react back again to ozone. Only <3% initialise the formation of the most important radical in the atmosphere: OH
If two molecules or atoms A and B collide and form a third molecule C, there is a third partner M necessary, in order to remove some excess energy. This partner (ususally nitrogen N2) does not react itself.
|
|
 |
 |
|
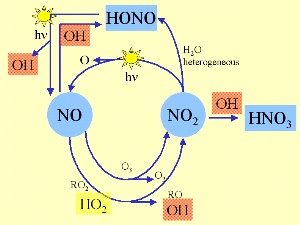
3. OH and the nitrogen oxide cycle
Scheme by Elmar Uherek
Please click to enlarge (90 K)
|
|
 |
How is OH formed?
OH governs atmospheric chemistry during the day, because its formation primarily depends on the radiation from the sun. The initial reaction (above) is the cracking of ozone by sunlight (photolysis) taking place at wavelenghts less than 310 nm, followed by the reaction of the formed O atom with water. This is why a basic amount of ozone in the troposphere is essential for its chemistry, although too much is not healthy.
Other sources of OH are the photolysis of nitrous acid HONO, hydrogen peroxyde H2O2 and peroxy methane CH3OOH, the reaction of NO with the hydroperoxy radical HO2 or the reaction of alkenes with ozone. The scheme on the left shows, in which way OH is intertwined with the daytime reaction cycles of nitrogen oxides.
|
How much OH is formed?
Since OH is an extremely reactive radical it reacts as soon as it is formed. It's lifetime is about a second or less. This means the concentration is extremely low, in the range of 1x105 to 2x107 molecules cm-3. For sea level pressure this is a mixing ratio of 0.01 - 1 ppt (pmol/mol).
Since the formation depends on the availability of water vapour, the concentration of OH tends to decrease with increasing altitude (cooler and dryer air).
|
 |
 |
 |
|
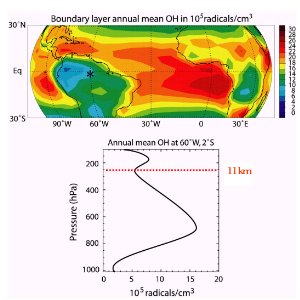
4. Zonal distribution of OH
A pressure of 250 hPa is reached at roughly 11 km altitude (tropopause in the mid latitudes). Which are the altitudes for 1000, 800, 600 and 400 hPa? Use the diagramme of the pressure altitude relationship!
source: presentation J. Lelieveld - MPI Mainz 2003
Please click to enlarge (80 K)
|
|
But it decreases in particular with the latitude because not only the water vapour concentration decreases but also the intensity and the duration of sunlight towards the poles.
How does OH react?
The image on the right shows an interesting effect over the tropical rainforest. The OH concentration decreases near the ground. What is the reason? Many organic compounds, first of all isoprene, are emitted by the forest and react with OH. Therefore a strong OH removal occurs close to the ground. It is consumed in chemical reactions. OH has a strong tendency to abstract an hydrogen atom from organic compounds RH whenever possible and to form water H2O. In the next step the radical R reacts with oxygen O2 and forms organic peroxides which are for example essential for the ozone formation cycle. reacts with oxygen O2 and forms organic peroxides which are for example essential for the ozone formation cycle.
|
 |
 |
 |
|
5. OH distribution in the tropics
top: global distribution in tropical regions
below: profile over the Manaus rain forest station (Brazil)
source: presentation J. Lelieveld MPI Mainz 2003
Please click to enlarge (80 K)
|
|
|
However, worldwide OH does not react first of all with organic compounds from forests. Organic gases contribute with 30% to the removal of OH plus another 15% for methane, the most important and smallest of the organic molecules. The main gas which reacts with OH is carbon monoxide (40%) and the remaining 15% react with ozone O3, hydroperoxy radicals HO2 and hydrogen H2.
|
 |
 |
|
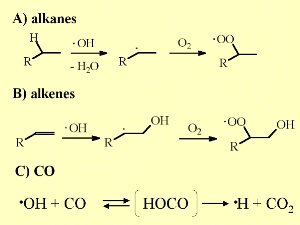
6. Important OH reactions in the troposphere
Please click to enlarge (45 K)
|
|
 |
Reacting with small alkenes, a special class of organic compounds, OH tends to add to the double bond as long as the saturated rest is not much bigger and H abstraction statistically favoured. Also here a formation of peroxides occurs.
OH is able to oxidise carbon monoxide CO to carbon dioxide CO2. As we saw CO and methane CH4 are the main sinks of OH. Other reactive organic compounds are only available in traces of some ppt, while CO reaches average levels of 120 ppb in the northern hemisphere (more combustion processes) and 60 ppb in the southern hemisphere.
|
Although OH is the most important oxidant in the atmosphere, its night time concentration is close to zero because radiation is needed for the formation. This is why during dark periods and during the night, the chemistry of nitrate NO3 and also ozone O3 becomes more important.
|
 |
 |
 |
|
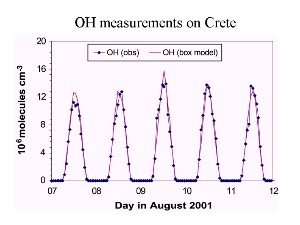
7. Time profile of OH concentrations over several days.
Source: Presentation J. Lelieveld MPI Mainz 2003
|
|
* The mixing ratio ppb or ppm (= 1 molecule among 1 billion molecules, or 1 molecule among 1 million molecules) is often used in scientific publications as well as in other literature on atmospheric and climate science. We also use it here in the Climate Encyclopaedia. However, more correct is the unit 1 nmol/mol (= 1 ppb) or 1 µmol/mol (= 1 ppm). Because the quantity of molecules n is measured in the unit mol.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
scientific reviewer: Dr. Mark Lawrence - Max Planck Institute for Chemistry, Mainz 2004-05-05
educational proofreading: Michael Seesing - Uni Duisburg - 2003-07-02
revised and last published: 2004-05-07
|
|
 |
|









