|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Basics |
Ozone smog
Ozone smog is part of big city air pollution. Ozone is formed in an intricate process involving nitrogen oxides, ozone formation and ozone degradation. It is an example of how various processes in the atmosphere are linked together.
|
|
|
|
|
 |
 |
 |
|
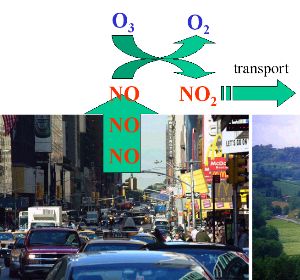
1. NOx emission in the city
image: Elmar Uherek, Foto © FreeFoto.com
Please click on the image in order to enlarge! (80 K)
|
|
 |
What happens in the city?
Lets make the example simple and assume nearly all nitrogen oxides come from combustion processes in cars. NO rich air rises from the streets and highways. The first reaction taking place is not a formation but a consumption of ozone, because NO is oxidised by ozone and forms nitrogen dioxide NO2. Indeed, directly over the streets and near the highways ozone concentrations are often very low. During ozone smog periods the ozone concentrations in the town can be lower than in the rural areas around. The plumes of NOx rich air are transported with the wind to the countryside.
|
 |
 |
|
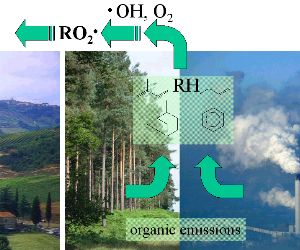
2. organic emissions from forests and industry
image: Elmar Uherek, foto © FreeFoto.com
please enlarge! (120 K)
|
|
 |
Where do organic compounds come from?
The second partner needed in the ozone formation cycle are organic peroxides. Where do they come from? Organic molecules are emitted from forests and other plants but also from human sources (e.g. solvents or fuel at filling stations). We show the structure of a few organic compounds, which are abbreviated with RH. These compounds are chemically attacked in the air. The typical reaction favoured by sunlight is the reaction with the hydroxy-radical  OH and after that an addition of an oxygen molecule. The result is a peroxy-radical RO2 OH and after that an addition of an oxygen molecule. The result is a peroxy-radical RO2 . R stands for an organic rest, the part of the molecule which is not involved in the reaction. Radicals have a single electron, which is symbolised by the dot and makes them very reactive. . R stands for an organic rest, the part of the molecule which is not involved in the reaction. Radicals have a single electron, which is symbolised by the dot and makes them very reactive.
|
 |
 |
|
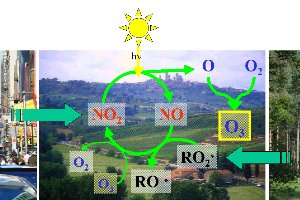
3. Formation of ozone smog
image: Elmar Uherek, Foto © FreeFoto.com
Please click the image in order to enlarge! (115 K)
|
|
 |
When are the best conditions for ozone smog?
Over the rural areas around the town the ozone formation cycle can begin.
1) Nitrogen dioxide NO2 is photolysed by the sun and forms O-atoms and nitrogen oxide NO.
2) O-atoms react with the molecular oxygen in the air and form ozone (O3).
3) Nitrogen oxide NO reacts with peroxy-radicals RO2 and forms NO2 again. and forms NO2 again.
4) Some ozone is consumed by NO depending on the competing concentration of peroxy-radicals RO2 . .
|
|
In the end the peroxy-radicals are consumed and ozone is formed while the nitrogen oxides are always recycled. This can only happen if
a) enough sunlight is available for efficient photolysis (hot sunny days)
b) the mixture of peroxy-radicals and nitrogen oxides favours the ozone formation.
|
 |
 |
|
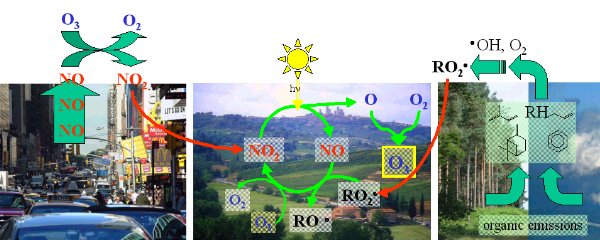
4. The complete ozone oxidation cycle
Full size: 100 KB, 960 px width
image: Elmar Uherek
|
|
 |
|
|
If no nitrogen oxides are available, the reaction cycle cannot take place.
If too much nitrogen oxides are available the excess of nitrogen oxide NO reacts not only with the peroxy-radicals but removes the ozone again.
If no sunlight is available NO cannot be recycled again and the formation of peroxy-radicals is not sufficient.
Usually in clean air the amount of nitrogen oxides is not high enough for a severe ozone smog period. But if man emits too much of them it can happen. A comparable situation can be given in a plume of vegetation fires, where nitrogen oxides are formed in the flames.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute, Mainz
scientific reviewer: Dr. Rolf von Kuhlmann - Max Planck Inst. for Chemistry, Mainz
educational proofreading: Michael Seesing - Univ. of Duisburg - 2003-07-02
revised and last published: 2004-04-30
|
|
 |
|









