|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more |
Understanding tropospheric ozone abundance
Where does ozone come from? By which mechanisms is the formation and removal governed? We try to get a deeper understanding, why the appearance of ozone in our air cannot be described in a simple way.
|
|
|
|
|
 |
Ozone control by nitrogen oxides and organic compounds
We learned about the diverse characteristics of ozone in the lower atmosphere in Unit 3 Basics. Have a look again on the chapter 'ozone smog' and the scheme below. |
 |
 |
|
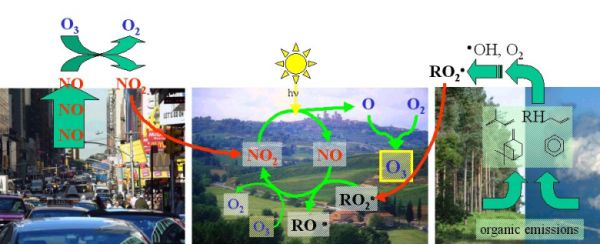
1. How ozone smog is formed. - Scheme: Elmar Uherek
Click on the image to enlarge! (100 K)
|
|
High ozone concentrations in and around urban areas appear, if hot days, traffic and emissions of organic gases (so called VOC = volatile organic compounds) come together.
|
The presence of nitrogen oxides and sunlight (hv*) alone does not necessarily lead to ozone formation, because ozone reacts back with the formed NO. The consequence of such a cycle is, that there is a high conversion but no net production or depletion. We call this a null cycle. It is often found in the atmosphere, also for other compounds.
|
 |
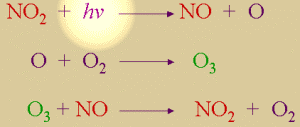 |
 |
|
2. a) Cycle of ozone formation and consumption. There is no net production or depletion.
|
|
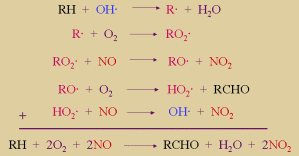
However, in the air are also volatile (=gaseous) organic compounds (VOC). In chemical reactions they are often abbreviated RH. They react with  OH radicals, form peroxi radicals RO2 OH radicals, form peroxi radicals RO2 and these radicals oxidise NO. Since this reaction competes with the ozone consumption more ozone may be formed than depleted. and these radicals oxidise NO. Since this reaction competes with the ozone consumption more ozone may be formed than depleted.
*hv stands for sunlight, since the quantum mechanical expression of the light energy is E = hv
|
 |
 |
 |
|
2. b) The peroxy chemistry of organic compounds RH competes with ozone for the NO.
|
|
 |
 |
|
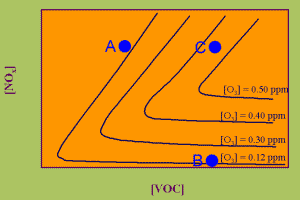
3. Ozone isoplethes - The graph shows the dependence of the formation of high ozone concentrations on the ideal mixture of volatile organic compounds (VOC) and nitrogen oxides (NOx).
Images 2+3 are adopted from a lecture of Mike Hannigan, Univ. of Colorado, Boulder
|
|
 |
In a diagram of so called isopleths you see black lines of the same ozone concentration. If more or less ozone is formed, strongly depends on the right mixture of nitrogen oxides NOx and volatile organic compounds VOC (=RH). If much nitrogen oxide is in the air, but not enough VOC, no ozone may be formed (blue point A). It even can be consumed as sometimes in the city centre. If a high level of VOC is available, but nearly no NOx, the ozone level is low as well (point B). Only if the right mixture is given, both in rather high levels, also ozone levels increase (point C). Since the ozone level quickly adopts to local conditions, ozone concentrations vary depending on daytime, sunlight and local emissions. It is difficult to calculate a global average concetration as for methane or carbon dioxide.
|
Other sources
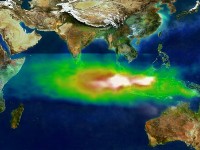
Urban traffic is not the only source of ozone, since urban traffic is not the only source of nitrogen oxides, which are important for ozone formation. They can come from other combustion processes as e.g. in vegetations fires. The visualisation on the right shows in white the smog and from green to yellow and red increasing ozone concentrations in the plume of the big fire in Indonesia 1997. You will read more about other processes going on in vegetation fires in the next text.
Lightning is another source of nitrogen oxides. Once high ozone concentrations have been observed in Australia and researchers were looking for human sources. However, finding out where the wind came from, it became clear, that thunderstorms and heavy lightning over South Africa was the reason.
|
 |
 |
 |
|
4. Smog plume from vegetation fire at Indonesia
Image courtesy NASA GSFC Scientific Visualization Studio, based on data from TOMS
Please click to enlarge (50 K)!
|
|
 |
 |
|
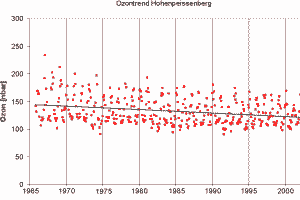
5. Trend in the monthly ozone abundance measured at the Hohenpeissenberg Observatory
Image: courtesy of the German Weather Service
|
|
 |
Measuring ozone
Seeing the plume image from Indonesia and the Indian ocean you may ask from where we have the information about ozone concentrations. Indeed there are not so many measurement points in Indonesia and certainly not in the ocean. Ozone is observed from space by instruments installed on satellites. However, working with satellite data, we have to take into account, that from space usually the whole atmosphere is observed. Look on the trend on the right measured at the German Hohenpeissenberg Observatory. Does it mean, that ozone in Germany is decreasing, although we learned that ozone in the troposphere has been increasing nearly everywhere?
|
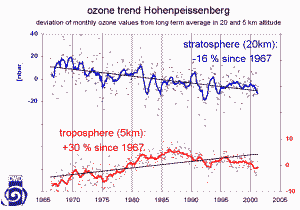
No. Ozone trends depend very much on the locations and there are high variations. However, we cannot estimate ozone trends either from the global view of satellites or the local view of single ground stations. The data separated for two different altitudes on the right show, that at the same place there are different trends depending on the altitude. We know that ozone is decreasing in the stratospheric ozone layer and we learn much more about it in the topic 'Higher atmosphere'. Satellites see both, the troposphere and the stratosphere at the same time. But ozone distributions are too complicated to be understood without careful separation into different phenomena.
|
 |
 |
 |
|
6. Ozone data from Hohenpeissenberg separated for two altitudes, 5 km in the troposphere and 20 km in the stratosphere.
Image: courtesy of the German Weather Service
|
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
scientific reviewer: Dr. Rolf Sander - Max Planck Institute for Chemistry, Mainz 2004-05-18
last published: 2004-05-24 |
|
 |
|









