|
|
 |
|
|
|
 |
| |
|
|
 |
Higher Atmosphere
Read more |
Stratospheric Ozone Chemistry
It was primarily after 1980 that our knowledge about stratospheric ozone chemistry grew a lot. The reason was the discovery of the ozone hole in 1985. The following two sections give an overview of the stratosphere's chemistry in its historical frame.
|
|
|
|
|
 |
Discovery of ozone and first measurements
Ozone research is a rather old branch of atmospheric sciences. In 1840 the gas was baptised 'ozone' (the smelling) by the chemist Christian Friedrich Schönbein, who discovered that this substance was formed during electric discharges. Very soon it has been found out, that ozone is a natural part of the air. The first method for measuring this gas has been developed by Schönbein himself, but very soon it was improved at the Mt. Souris Observatory in Paris. From there comes the first series of data (1876-1910) which is today the best guess for preindustrial concentrations in the boundary layer.
|
 |
 |
 |
|
1. Christian Friedrich Schönbein
source: Webpage Swiss Academy of Science techniques
|
|
 |
 |
|
2. The Dobson Spectrometer
Courtesy of: Ulf Köhler, DWD Hohenpeissenberg
Please click to enlarge! (130 K)
|
|
 |
In 1879 it was discovered that the spectrum of the sun significantly declines in the UVB region at the Earth surface and in 1880 it was found, that ozone is a strong absorber in this range and could be responsible for it. The amount of ozone available in the lower troposphere however, could not explain the UVB decline. Therefore assumptions have been made, that most of the ozone must be formed in higher layers of the atmosphere. The key research was done by Gordon Dobson in the twentieth of the 20th century. He developed the Dobson-spektrometer which has been used since 1929 for the measurements of the total ozone column and is nowadays gradually replaced by more modern methods, but still in use.
For more details how a Dobson spectrometer works, please click  HERE! HERE!
|
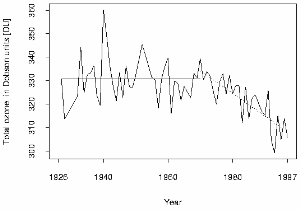
One of the first six Dobson spectrometers has been used at Arosa in Switzerland by Paul Götz and from there we have the longest series of measurements of the total ozone column in the world. The trend shows, that also over Europe the ozone layer became thinner. Values of less than 300 DU have been measured during the last years in summer at Hohenpeissenberg, a critical limit which makes better sun protection necessary. Also 200 DU in spring during the time of the ozone hole over the Northern hemisphere in March are very dangerous.
In the thirtieth of the 20th century Götz showed that the maximum of the ozone concentration is very likely below 25 km. The ozone layer was more or less located and its thickness measured.
|
 |
 |
 |
|
3. The Arosa ozone series
source: ETH Zürich
|
|
 |
 |
|
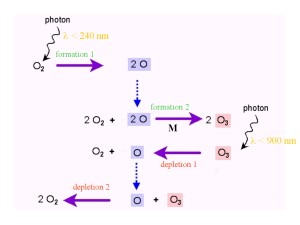
4. The Chapman reactions (image changed!)
scheme by EU
Please click to enlarge! (40 K)
|
|
 |
The Chapman reactions
But how is the ozone formed and removed again? Also in 1929 and 1930 S. Chapman published the theory of ozone formation and depletion. The reactions are still valid and called the 'Chapman cycle' or the 'Chapman reactions'.
Oxygen and ozone are transformed into each other. The bonds are broken by photolysis due to solar radiation. In order to break the bond in O2 the energy of the sunlight has to be higher (wavelength shorter than 240 nm), than for ozone (wavelength shorter than 900 nm). Formation and depletion are in equilibrium and the net result is a 'zero' reaction:
3 O2 -> 2 O3 and 2 O3 -> 3 O2
|
Absorption in the UV
It depends on the absorption of a molecule, if a bond can be broken by pure light. Each molecule absorbs energy in a certain range of the electromagnetic spectrum. Oxygen absorbs in the highly energetic UV-C range, ozone in the slightly less energetic UV-B range. Longer wavelength partially pass the atmosphere and reach the Earth surface.
5. Absorber spectra (on the right):
The image shows the combined absorption spectra of the main absorbers of solar radiation in the upper atmosphere. It indicates the altitudes, to which the respective parts of the sunlight go down. Light of a wavelength less than 200 nm is already blocked in the ionosphere and mesosphere by nitrogen N2, O-atoms and oxygen O2. Light between 200 and 320 nm goes further down to the stratosphere (below 50 km), where it is mostly absorbed by ozone O3. Finally light of more than 320 nm reaches the Earth surface.
|
 |
|
|
A small fraction of UV-B light however reaches the Earth surface allowing the formation of OH radicals which clean the troposphere. This fraction is also critical for biological implications like sunburn or damage of the DNA.
|
Ozone depletion by radicals
It became more and more clear that the measured concentrations of ozone can not only be explained by the simple Chapman reactions. From 1970 on Crutzen, Molina, Rowland (Nobel prize 1995) and other scientists developed the theory of an involvement of nitrogen oxides and halogen radicals in the ozone chemistry. Molina and Rowland discovered already in 1974, that chlorofluorocarbons destroy ozone.
|
 |
 |
 |
|
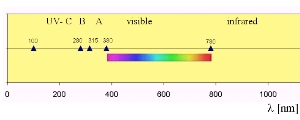
6. UV light in the electronmagnetic spectrum
Apart from the one given here, there are several definitions of UV light ranges, e.g. IPCC defines UV-A light from 315-400 nm.
composed by: Elmar Uherek
Please click to enlarge! (60 K)
|
|
 |
 |
|
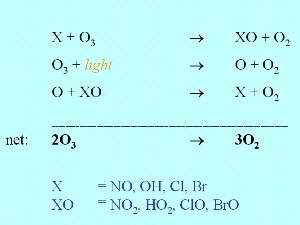
7. Chemical ozone depletion
Please click to enlarge! (40 K)
|
|
 |
Ozone is not only depleted by photolysis but also by the reaction with a compound X , which can be nitrous oxide NO, the hydroxyl radical , which can be nitrous oxide NO, the hydroxyl radical  OH or an halogen radical as Cl OH or an halogen radical as Cl or Br or Br . There are also some other radicals of minor importance which react in the same way. . There are also some other radicals of minor importance which react in the same way.
Since the release of halogen containing compounds by human acitivity was known, a slight decrease of the ozone concentration was predicted by some scientists. However, the idea of the processes in the stratosphere was not yet complete and a that drastic reduction as in the ozone hole over Antarctica has not been expected, before it was detected in 1985.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
scientific reviewing: Dr. Christoph Brühl - Max Planck Institute for Chemistry, Mainz
educational proofreading: Michael Seesing - Uni Duisburg - 2003-08-07
last published: 2004-05-11
|
|
 |
|









