|
|
 |
|
|
|
 |
| |
|
|
 |
Higher Atmosphere
Read more |
Ozone hole and global warming
When environmental problems are discussed, people tend to bring together the ozone hole and global warming. A fact is: The ozone hole is not a direct consequence of global warming, global warming is not a direct consequence of the ozone hole. But since nearly everything in the climate system is linked, also here are linkages.
|
|
|
|
|
 |
 |
 |
|
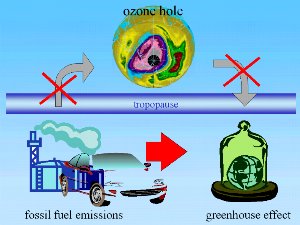
1. There is no direct link between ozone hole and greenhouse effect. Emissions from fossil fuel burning (CO2) lead to the greenhouse effect but not to the ozone hole.
Image by Elmar Uherek
Full size: 75 K
|
|
 |
The big misunderstanding
Many people have never heard about the climate system in school and this branch of research is rather new. Therefore it is not surprising, that the two major challenges dominating the headlines, are often mixed up: global warming and the ozone hole.
The fact, that more and more cars circulate on our planet and burn gas and that more and more energy from fossil fuel products is consumed in industry and households, was not the reason for the formation of the ozone hole.
The formation of the ozone hole, on the contrary, does not lead to a global warming.
Both, the ozone hole and global warming, are in their main causes different phenomena of human influence on the atmospheric system!
But there are more indirect links ...
|
Where are the links?
Global warming is a phenomenon which has its main impacts on human life in the troposphere. The atmospheric layer next to the Earth surface, our direct environment, warms up.
The ozone hole is formed in the stratosphere. Ozone, acting as a shield against UV-radiation in an altitude of about 15-40 km, is destroyed.
|
Chlorofluorocarbons CFCs play a role in both processes.
On the one hand, in the troposphere, they absorb infrared light in the atmospheric window. Therefore, they have a strong global warming potential. This absorption capability is a consequence of their optical behaviour.
On the other hand, in the stratosphere, they are a source of chlorine radicals, leading in a chain reaction to the depletion of ozone. This is because the CFC molecules contain chlorine atoms. Since CFCs are very stable the chlorine atoms are not set free in the troposphere but in the stratosphere by cleavage due to strong UV-light.
Other greenhouse gases as CO2 or methane do not play a comparable role in ozone depletion.
The ozone hole changes the radiation budget.
|
 |
 |
 |
|
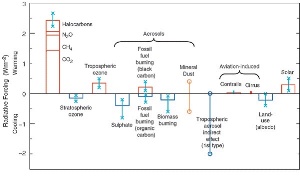
2. The radiate forcing diagramm shows to which extend which factors favour (positive columns) or counteract (negative columns) the greenhouse effect. The two columns on the left show, that halocarbons are greenhouse gases and contribute so the anthropogenic greenhouse effect. Stratospheric ozone loss however slightly counteracts the greenhouse effect and leads to a cooling.
Source: IPCC TAR 2001
Please click to enlarge! (50 K)
|
|
|
Since ozone holds back the UV-B radiation from the Earth and leads to a warming in the stratosphere, it can be assumed, that changes in the total radiation budget of the Earth appear, if ozone disappears. Indeed, this is the case. But the depletion of ozone does not lead to a further warming but to a slight cooling.
|
 |
 |
|
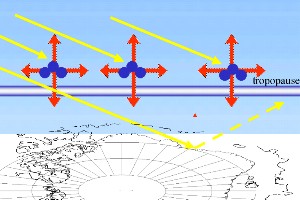
3. a) An intact ozone layer holds back most of the solar UV radiation and transfers its energy into heat radiation.
|
|
 |
The impact of the ozone hole on the radiation budget
It is not directly obvious that the ozone hole shall promote the so called 'negative radiative forcing', i.e. lead to a cooling of the troposphere and therefore counteract the greenhouse gases. Our first thoughts may be: If the ozone layer becomes thinner, more ultraviolet radiation, primarily in the UV-B range, reaches the Earth's surface. This means more energy from the sun. Certainly this is true and the main reason for the increasing risk of skin cancer. However, there is an counteracting effect. |
 |
 |
|
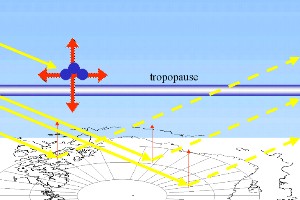
3. b) The depleted ozone layer (ozone molecules in both pictures in blue) absorbs only a small fraction of the UV radiation of the sun and in the lower stratosphere also less IR radiation from Earth. The transfer into heat radiation is much smaller. The not absorbed sunlight reaches the Earth but a large fraction is scattered back, in particular from the white ground of the Antarctic continent. Only a small fraction is transferred into infrared radiation.
Images by Elmar Uherek
Please click images to enlarge them! (65 K)
|
|
 |
(!changes made!) As we know, the absorption of UV-light by ozone molecules causes a warming in the stratosphere. The heat emitted from stratosphere is also transferred to the troposphere and has there a warming impact. Also, in particular in the lower stratosphere, ozone still acts as greenhouse gas and absorbs infrared light coming from the Earth. The reduction of this heat transfer from both sources, IR and UV-light, to the troposphere overcompensates the additional energy from the solar radiation. The latter is partiallly backscatterd to the space by the Earth's albedo (clouds, ice shields, light ground). Backscattering is exceptionally strong over the Antarctic region, where the strongest ozone depletion occurs. The snow and ice covered ground has an albedo of 0.6 to 0.8 (60-80% of the radiation are scattered back), compared e.g. to a value of 0.1 for the oceans.
|
The impact of global warming on the ozone hole
In the next section 'stratospheric cooling' we will see, that global warming of the troposphere causes a cooling in the stratosphere and therefore promotes ozone depletion, perhaps also over the northern hemisphere.
|
About this page:
author: Dr. Elmar Uherek - Max Panck Institute for Chemistry, Mainz
scientific reviewer: Dr. Christoph Brühl - Max Planck Instite for Chemistry, Mainz
educational proofreading: Michael Seesing - Uni Duisburg - 2003-08-07
last published: 2003-05-11
|
|
 |
|









