|
|
 |
|
|
|
 |
| |
|
|
 |
Clouds & Particles
Basics |
Particles: where do they come from?
Aerosols are small solid or liquid particles suspended in the atmosphere. Their sizes vary from a few nanometers (0.000000001 meters) to almost 100 micrometers (0.0001 m, the thickness of a hair), that's why we can not see them generally.
|
|
|
|
|
 |
Aerosols originate both from natural and man-made (anthropogenic) sources . They can be directly emitted as particles (primary aerosols) or they can also be the result of chemical reactions (secondary aerosols).
Sources of primary aerosols
Aerosols can be of natural or anthropogenic origin.
|
Marine origin
Sea salt aerosols are generated by the sea spray of waves at high wind speed (the largest particles, that are typically enriched with sodium chloride), and by the bursting of entrained air bubbles during whitecap formation. Emissions of aerosols in the atmosphere by the oceans are around 1,3 billions tonnes per year !
|
 |
 |
 |
|
1. Source: Ph. Osset, www.mesvoyages.net
|
|
 |
 |
|
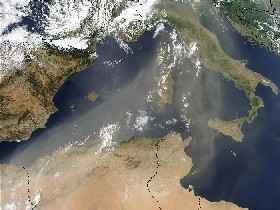
2. A river of Saharan dust flows over Mediterranean sea and Italy on July 16, 2003.
Source: NASA/Seawifs
|
|
 |
Mineral origin
The wind can pick up surface particles especially when soil is dry, and without plant cover, and carry these small grains on great distances. These particles consist of materials derived from the earth's crust, and are therefore rich in oxides of iron, calcium, and aluminum. Half of the total emission of mineral particles on the Earth is originated from the Sahara desert. |
Volcanic origin
Volcanic erupts inject enormous quantities of gases and aerosols in the atmosphere. Contrary to the other aerosols sources, ash plume are so high that particles and gases can penetrate the stratosphere: the Pinatubo volcanic plume has reached 40 km height!
Particles that enter the upper atmosphere are not easily washed-out and volcanic material remains at the level injected for a long time (sometimes several years). The gases emitted by volcanoes also produce aerosols . All these stratospheric particles have a strong impact on climate (see more details in the "More" section).
|
 |
 |
 |
|
3. St Helens erupt on May 18, 1980. Source: NASA
|
|
|
Biogenic origin
Particles that are produced by action of living organisms are called biogenic particles. Primary aerosols can be pollens, fungi, bacteria, viruses... Another origin is forest fires that release particulate matter in the atmosphere. For example in some areas of Malaysia in 1997, smoke from fires has led to 15 times higher levels in particle pollution than normal levels, during several weeks.
Cosmic origin
Some matter from the solar system enters our atmosphere. Most of it is burn in the upper part of the atmosphere (causing the "shooting stars" we see), but some particles strike the earth's surface. These particles, smaller than 0.5 mm, are called micrometeorites. Scientists estimate that at least several thousands of tons per year of this cosmic matter reach the ground.
|
Anthropogenic origin
Particles emitted by human activities are called anthropogenic aerosols. Anthropogenic sources produce both coarse and fine particles. Dust from roads and construction sites (like cement works) produce primarily coarse anthropogenic particles. Smaller particles like carbon containing aerosols are generated through fossil fuel combustion in power generation, vehicles, residential heating ...
|
 |
 |
 |
|
4. Source: J. Gourdeau
|
|
|
Finally, even if they do not have an impact on climate system, do not also forget all the particles you breath in indoor environments, like dust mites, fibers, insect sprays and asbestos, that can be very dangerous for human health.
|
5. Scanning electron or transmission electron microscope images. From the left to the right: desertic particle (source: A. Gaudichet, LISA); hibiscus pollen (source:http://uq.edu.au/nanoworld); ash particle from the eruption of Mount St. Helens (source:http://volcanoes.usgs.gov); indoor moulds (source:M. Boissier, CSTB); soot particle (MPI for Chemistry Mainz). |
Secondary aerosols
As you've just seen, aerosols can be directly produced by many sources (marine, mineral, volcanic, biogenic, anthropogenic). A solid material is at the starting point of the source process. But particles can also be the result of "gas-to-particle-conversion". It meens, new particles may form because molecules gather together, and these molecules are too small to be counted as particles yet (this process is called nucleation). Gases can also condense on pre-existing particles to form bigger aerosols. By the way, particles issued from gas-to-particle conversion are fine (less than 1 Ám), compared to coarse particles (for example, mineral dust, pollens or sea spray have diameters 10 Ám or more).
|
 |
 |
|
6. Rose. Source: www.freefoto.com
|
|
 |
Vegetation also emit gases, named Volatil Organic Compounds (your nose detects some of these VOCs when smelling a flower) (for more details see plants emissions in the Lower atmosphere unit ). These biogenic gases can form new particles (secondary aerosols). |
|
The majority of secondary natural aerosols result from reaction of sulfur gas emissions. In the marine environment, most sulfur is emitted in the form of DMS by phytoplancton. Reactions of DMS with atmospheric compounds form sulfur dioxide (SO2); on land, decaying vegetation and animals produce natural H2S (that smell like rotten eggs...yuk!); volcanoes directly release SO2. This gas can further react to form sulfate aerosols particles.
Natural sources produce also carbon containing gases that yield to aerosols.
|
 |
Man-made annual rate of SO2 emissions has increased from 10 millions tons per year in 1860 to 150 millions tons per year in the eighties. Therefore, anthropogenic emissions now exceed natural emissions of sulfur-containing gases, even if atmospheric SO2 levels are now falling because of international legislation.
Humans also produce more and more nitrogen species that give nitrate aerosol.
The reaction of some anthropogenic compounds, issued from petroleum combustion processes and biomass burning, results in carbonaceous aerosols, that pose a health hazard. |
About this page...
Author: Justine Gourdeau LaMP Clermont ferrand/France
Scientific reviewer: Pr Guy Cautenet, LaMP, France.
date of generation: 2003-09-02; last published: 2004-04-21. |
|
 |
|









