|
|
 |
Attention: Use a fume hood for this experiment.
(Sulfurdioxide is toxic.[T; R: 23-34; S: 9-26-36/37/39-45])
You need these things for the experiments:
|
| 1 |
fireproof plate (crucible) (a) |
|
filter paper |
| 1 |
large glass funnel (b) |
|
fuchsine |
|
2 |
small wash bottles (or test tubes with...) (c) |
|
sulfur |
|
1 |
wash bottle (middle szied) (e) |
|
activated carbon (granulated) |
|
1 |
water-operated vacuum pump (f) |
|
lime water (conc. Ca(OH)2 (aq)) |
| |
|
|
CaCO3 - powder |
| |
hoses |
|
water |
|
1 |
(stop) watch |
|
diluted hypochloric acid
[C; R: 34-37; S: 26-45] |
|
 |
 |
|
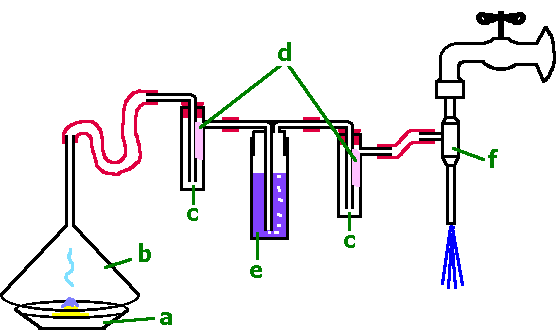
picture 1: experimental setup: flue gas desulfurization
a, b, c, e, f: see table above
d: filter paper of the same size soaked with a heavy diluted solution of fuchsine.
© 2004 Seesing, Tausch Universitšt-Duisburg-Essen, Duisburg
|
|
| E1 |
First arrange the experimental setup like it is shown in picture 1. At the first experimental step (a)) the wash bottle (e) stays empty. For every experiment soake 3 small pieces of filter paper (approx. 20 x 30mm) with heavy diluted solution of fuchsine. (approx. 0,02g Fuchsin in 100mL of water). Put two of these "Indicator-papers" in the wash bottles as it is shown in the picture 1 (d). A third one is used as a reference. Ignite approx. a spoonful of sulfur (a). (The amount of sulfur should burn about 5 minutes.) Suck the product sulfur dioxide with a water-operated vacuum pump softly through the equipment. Especially pay attention to the color of the filter papers. If the indicator paper of the first wash bottle is nearly decoloured (after approx. 5 minutes) stop the experiment. Repeat this experiment in the same way, but fill 3/4 of the middle wash bottle (e) with b) granulated activated carbon and c) a saturated solution of calcium hydroxide Every experiment needs three fresh made indicator papers. |
|
T1 |
Note your observations using headwords. Especially take care about the decolourization of the different placed indicator papers. |
|
E2 |
Acidify the content of the middle washbottle of experiment c) up to the pH <5 by adding drops of diluted hypochloric acid. |
|
T2 |
Note your observations. |
|
T3 |
Are only the solution of calcium hydroxide together with sulfur dioxide sufficient in order to form gypsum?
Set up the equation of the overall reaction from sulfur dioxide to gypsum. |
|
|
T4 |
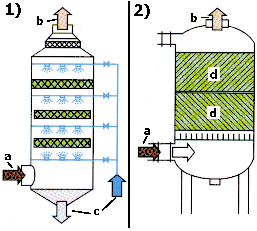
In which part of the experiment E1 takes an adsorption, in which an absorption place?
Argue with the help of the terms definitions. (see also picture 2.) |
|
 |
 |
 |
|
absorption adsorption
solution in liquid attachement and
phase condensation at
the solid body
picture 2: methods of flue gas
desulfurization
a: crude gas; b: clean gas;
c: washing solution;
d: solid body
© 2004 Seesing, Tausch Universitšt-Duisburg-Essen, Duisburg
|
|
|
|
T5 |
Regard the products of the flue gas desulfurization at the experiment E1. If you are an owner of a coal dfired power plant, which method of desulfurization would you prefer?
Discuss the pro and contras of both methods. |
|
About this page:
Authors: M. Seesing, M. Tausch - Universitšt Duisburg-Essen, Duisburg / Germany
Rewiewer:
Last update: 2004-05-28 |
|
 |
|









