|
|
 |
|
|
|
 |
| |
|
|
 |
Urban Climate
Read more |
Origin of acid rain
Acid precipitation is a part of acid deposition, which can be wet or dry. The formation of acids in the air is caused by photochemical oxidation of SO2 and NOx, but significantly influenced by the presence of other substances, including volatile organic compounds (VOCs).
|
|
|
|
|
 |
|
A closer look at pH
In the chapter "What is acid rain?" (level "Basics", see link at the bottom of the page), we explained what pH scale is. The construction of the scale is based on the fact that pure water dissociates into hydronium ions H3O+ and hydroxide ions OH-

For this dissociation the law of mass action is valid. The constant Kw is called the ion product of water and it is always 10-14.
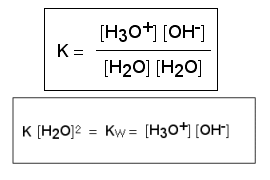
So, the law for any aqueous acid or base is pKw = pH + pOH = 14
In pure water c(H3O+) = c(OH-) = 10-7 mol/l and pKw = 7 + 7 = 14
We call this auto-dissociation of pure water.
|
|
Discovery of the acid rain problem
The phenomenon of acid rain was recognized already at the end of 17th century. In 1692 Robert Boyle published his book "A general history of the air", where he described it as "nitrous or salino-sulfurous spirits". The term "acid rain" was first used in 1872 by Robert Angus Smith (1817-1884), a scientist working in Manchester, in his book "Air and Rain: The Beginnings of Chemical Climatology". The acid rain problem was first discovered on larger scale in the 1960's. It was found that the acidity of the lakes in Scandinavia was increasing and the fish population was decreasing. Similar processes were occurring in North America. Additionally many forests were affected both in Europe and North America. That led to various scientific, political and technical actions.
|
|
Anthropogenic emissions as a source of acid rain
Apart from SO2 and NOx, that are emitted from combustion of fossil fuels, there are other substances that contribute to acid rain. They include chloride ions, ammonia and the VOCs. When gas-phase hydrochloric acid dissolves in raindrops, it forms aqueous hydrochloric acid (HCl (aq)). Chloride ions may arise from the oceans as sea-salt spray. The VOCs, which produce organic acids such as formic, acetic and oxalic acids via atmospheric oxidation and influence the chemistry that produces sulfuric and nitric acids, come mainly from automobile use in the urban areas, but their natural production by vegetation is ten times more significant than anthropogenic VOCs on a global scale. Most emissions of ammonia result from the soils, where artificial fertilizers are used as nutrients. The double role of ammonia is explained below.
|
|
Double role of ammonia
Ammonia (NH3) plays a double role in acidification. On one hand, it neutralizes in the atmosphere to a large extent the acid formed by oxidation of sulfur dioxide and nitrogen oxides, to form particulate ammonium (NH4+). Ammonia (NH3) as such is not an acid but a weak base. However, it reacts in the air with strong acids like sulphuric acid or nitric acid and then slightly acidic ammonium salts are formed, (NH4)2SO4, NH4NO3. They are less volatile, form particles and sink in the end to the ground or rain out. When NH4+ is deposited and enters the soil, nitrification can occur. The hydrogen ions from the atmospheric acid that was neutralized by NH3 in the atmosphere are released and additional acid is formed:
NH4+ + 2 O2 -> 2 H+ + NO3- + H2O
As a result, NH4+ deposition and subsequent nitrification leads directly to soil acidification. |
 |
 |
|
1. Sources and effects of acid rain and other forms of acid deposition.
Author: Sebastian Wypych
|
|
Deposition
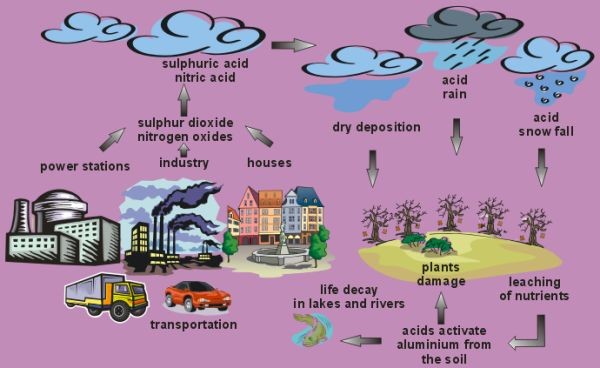
The processes by which chemical constituents are scavenged from the atmosphere to the earth's surface are called deposition. These processes include precipitation (wet deposition, such as rain or fog), as well as particle and gas deposition (dry deposition). When acid constituents are scavenged to the ground we call it acid deposition.
|
|
Wet deposition
The process by which chemicals are removed from the atmosphere and deposited on the Earth's surface via rain, sleet, snow, cloud water, and fog is called wet deposition. Acid rain is a result of many chemical reactions occurring in the air. The gases emitted to the air mainly due to combustion of fossil fuel in power stations and industry, that is, sulfur dioxide (SO2), nitrogen dioxide (NO2) and nitrogen oxide (NO), react with hydroxyl radicals and oxygen atoms forming acids. Acid particles are very hygroscopic (i.e. they absorb water easily), so they serve as condensation nuclei and enhance cloud formation. Finally, acid rain reaches the ground. The gases combined with fog may produce acid fog as well as acid snow etc.
|
 |
 |
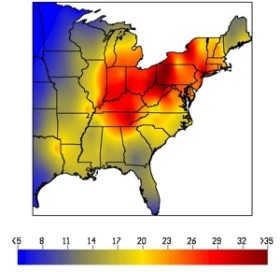
2. Trends in wet sulfate deposition (kg/ha) in the Eastern United States from NADP/NTN Monitoring Data. The image on the left shows data for the period 1989-1991, while the image on the right the data for 1995-1998. In the early 1990s, wet sulfate deposition was highest in a broad region of the Mid-western and eastern U.S. including the Ohio River Valley, western Pennsylvania, and the Mid-Appalachians (see 1989-1991). |
|
 |
 |
 |
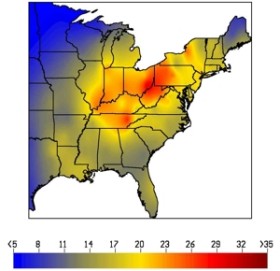
As a result of significant reductions in sulfur dioxide emissions beginning in 1995 (Phase I of the Acid Rain Program), total sulfur deposition in rain decreased significantly by up to 25% over a large area of the eastern U.S., unprecedented in magnitude and spatial extent (see 1995-1998).
Source: U.S. Environmental Protection Agency
http://www.epa.gov/airmarkets/cmap/
mapgallery/mg_wetsulfate.html |
|
|
Dry deposition
Although we meet with an acid threat on rainy days, acid deposition occurs all the time even on sunny days. The settling of acidic gases and particles out of the atmosphere is called dry deposition. It takes place when NOx and SO2 and their oxidation products reach the ground in the forms of gases, aerosols or dry particles. Then they react with water either in the ground surface, soil or rivers, but also damage plant leaves, buildings surfaces etc. About half of the acidity in the atmosphere falls back to the earth by dry deposition. The wind blows these acidic particles and gases onto buildings, cars, homes, and trees.
|
|
Dry-deposited gases and particles can be washed from trees and other surfaces by rain droplets. When that happens, the runoff water adds those acids to the acid rain, making the combination more acidic than a falling rain droplet alone. Dry deposition usually occurs near the emission sources, while wet deposition can take place even 1000 km away from the sources. Higher chimneys of the factories can emit pollutants into the middle troposphere, causing lower concentration of the pollutants near the surface, but affecting the larger areas by pollution. Rainwater acidity generally creates greater problems than acidity from dry deposition, assisted by chemical reactions that occur during long-range atmospheric transport from source regions.
|
About this page:
authors: Anita Bokwa - Jagiellonian University, Cracow, Poland and Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz, Germany
scientific reviewer: Kimitaka Kawamura - Hokkaido University, Sapporo, Japan - 2004-08-12
Educational reviewing: Michael Seesing - University of Duisburg, Duisburg, Germany
last update: 2004-12-17
|
|
 |
|









