|
 From sulphur dioxide to sulphurous acid From sulphur dioxide to sulphurous acid
If sulphur dioxide is dissolved in water, it forms a weak acid solution, sulphurous acid.
H2O + SO2 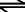 H2SO3 H2SO3
In water, sulphurous acid forms hydrated protons H+ (aq) and two sorts of anions are formed:
H2SO3 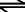 H+(aq) + HSO3-(aq) H+(aq) + HSO3-(aq)
Sulphurous acid forms a proton and a hydrogen sulphite anion
HSO3-(aq) 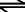 H+(aq) + SO32-(aq) H+(aq) + SO32-(aq)
Hydrogen sulphite gives a proton and a sulphite anion.
From each molecule of sulphurous acid two protons can be released. Therefore sulphurous acid is called a bi-protonous acid.
However, sulphurous acid is an unstable acid. Already, at normal room temperature, it decomposes into sulphur dioxide and water.
 Sulphuric acid Sulphuric acid
With the combustion of sulphur and during oxidative reactions in the air not only sulphur dioxide is formed but also sulphur trioxide. The amount formed of each of these gases depends strongly on the temperature, since sulphur trioxide decomposes into sulphur dioxide and oxygen at temperatures above 600°C:
2 SO3 -> 2 SO2 + O2 (at t > 600°C)
If sulphur trioxide dissolves in water, sulphuric acid is formed:
SO3 + H2O 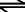 H2SO4 H2SO4
Sulphuric acid is also a bi-protonous acid which decomposes in water to yield two protons and an anion.
H2SO4 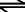 H+(aq) + HSO4-(aq) H+(aq) + HSO4-(aq)
In the first step sulphuric acid forms a proton and hydrogen sulphate anion.
HSO4-(aq) 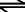 H+(aq) + SO42-(aq) H+(aq) + SO42-(aq)
Hydrogen sulphate decomposes to give another proton and a sulphate anion.
|







