|
|
 |
|
|
|
 |
 |
|
|
 |
Context 2: The sulphur cycle
|
Key words:
sulphur cycle, oxidation / reduction, sulphur and climate | |
|
 Sulphur cycle Sulphur cycle
We find many sulphur compounds on Earth. These include sulphur dioxide, elemental sulphur, sulphuric acid, salts of sulphate or organic sulphur compounds such as dimethylsulphide and even amino acids in our body. All these chemical compounds do not last forever. They are transported by physical processes like wind or erosion by water, by geological events like volcano eruptions or by biological activity. They are also transformed by chemical reactions. But nothing is lost. Changes often take place in cycles. Such cycles can be chemical cycles in which a sulphur compound A reacts to form B, B to C, C to D and D to A again. At the same time there are spatial / geographical cycles. One example is when sulphur compounds move from the ocean to the atmosphere, are transported to the land, come down with the rain and are transported by rivers to the ocean again.
|
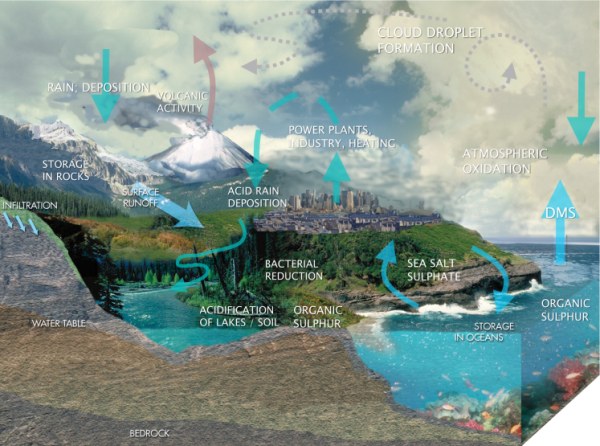 |
 |
|
1. The flow of sulphur compounds in our environment.
Scheme: Elmar Uherek, adapted and modified from an water cycle illustration of the Center for Space Research, Univ. of Austin, Texas
Please click the picture for a larger view! (150 K)
For a high resolution version (1.5 MB) please click HERE.
|
|
 Oxidation and reduction Oxidation and reduction
In chemical cycles, sulphur is usually oxidised in the air from organic sulphur or elemental sulphur to sulphur oxides like SO2 and SO3 ending up as sulphate in sulphate salts M(II)SO4, M(I)2SO4 or sulphuric acid H2SO4. The sulphate compounds dissolve very well in water and come down again with the rain, either as salts or as acid rain.
In chemical cycles oxidised compounds must also be reduced again. This process does not take place in the atmosphere but on the ground and in the oceans and is carried out in complicated chemical reactions by bacteria. The most important products are elemental sulphur, hydrogen sulphide (H2S), which smells awful and is very unhealthy, and organic sulphur compounds.
|
 Sulphur and climate Sulphur and climate
Sulphur compounds play a big role for our environment and the climate system. On the one hand they contribute to acid rain as described in this magazine. But they are also important for the formation of clouds as explained in the edition of January 2006. Finally, a lot of sulphur is brought into the air by volcanic eruptions. If it was a strong eruption, the emitted particles can go up to the stratosphere (9 - 12 km of altitude) and cool down half our planet by 1-2°C.
In the following scheme you can see factors which contribute to the sulphur cycle and how they are related to the climate system.
|
 |
 |
 |
|
2. The salts of the oceans also consist of sulphates. Some of the sulphates are brought as sea spray to the low layers of the atmosphere but do not have a strong influence on the climate.
Photo: © Rob Hartill with permission
|
|
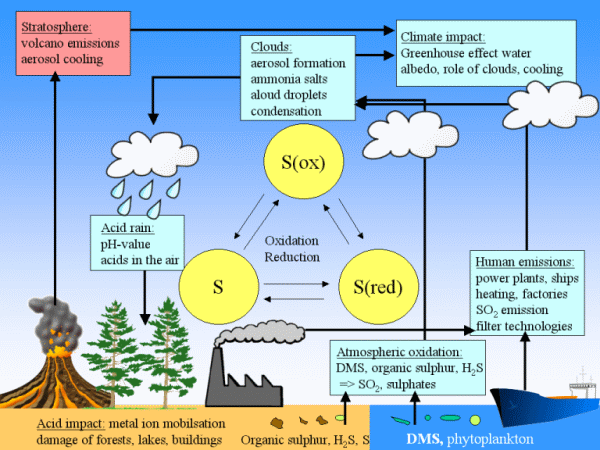 |
 |
|
3. Sulphur compounds play a role in our climate system in various ways. The scheme gives an overview. Click on it for a larger view. (90 K)
Scheme: Elmar Uherek
|
Author:
Elmar Uherek - Max Planck Institute for Chemistry, Mainz
English check: Mark Jacob, TU Freiberg
|
|
 |
|







